8.52057
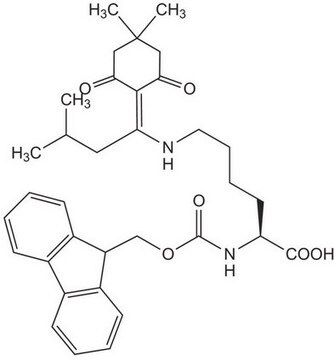
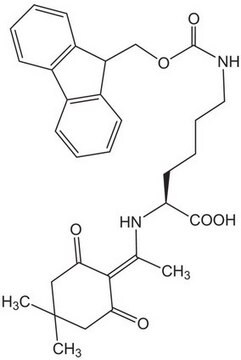
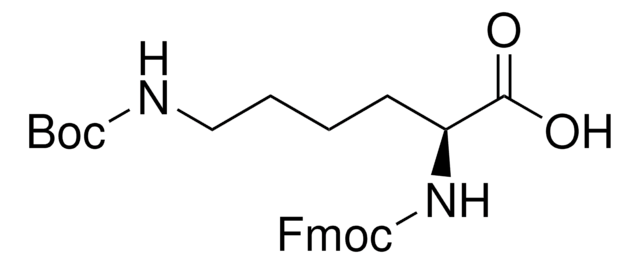
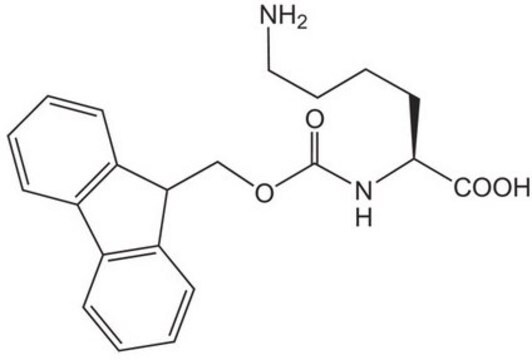
Fmoc-Lys(Dde)-OH
≥98% (TLC), for peptide synthesis, Novabiochem®
Synonym(s):
Fmoc-Lys(Dde)-OH, N-α-Fmoc-N-ε-1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl-L-lysine
About This Item
Recommended Products
product name
Fmoc-Lys(Dde)-OH, Novabiochem®
Quality Level
product line
Novabiochem®
Assay
≥90.0% (acidimetric)
≥97.0% (HPLC)
≥98% (TLC)
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
mp
80 °C (decomposes)
application(s)
peptide synthesis
functional group
amine
storage temp.
15-25°C
InChI
1S/C31H36N2O6/c1-19(28-26(34)16-31(2,3)17-27(28)35)32-15-9-8-14-25(29(36)37)33-30(38)39-18-24-22-12-6-4-10-20(22)21-11-5-7-13-23(21)24/h4-7,10-13,24-25,34H,8-9,14-18H2,1-3H3,(H,33,38)(H,36,37)/t25-/m0/s1
InChI key
AOHSSQNORWQENF-VWLOTQADSA-N
General description
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] B. W. Bycroft, et al. (1993) J. Chem. Soc., Chem. Commun., 778.
[2] B. Rohwedder, et al. (1998) Tetrahedron Lett., 39, 1175.
[3] N. Ahlborg (1995) J. Immun. Meth., 179, 269.
[4] B. W. Bycroft, et al. in ′Peptides, Chemistry, Structure & Biology, Proc. 13th American peptide Symposium′, R. S. Hodges & J. A. Smith (Eds), ESCOM, Leiden, 1994, pp. 727.
[5] J. Mack, et al. (2001) J. Peptide Sci., 7, 338.
[6] G. B. Bloomberg, et al. (1993) Tetrahedron Lett., 34, 4709.
[7] P. Dumy, et al. (1995) Tetrahedron Lett., 36, 1255.
[8] J. Eichler, et al. (1994) Pept. Res., 7, 300.
[9] C. G. Fields, et al. (1993) Biopolymers, 33, 1695.
[10] H. F. Brugghe, et al. (1994) Int. J. Peptide Protein Res., 43, 166.
[11] P. Hoogerhout, et al. (1995) Infection & Immunity, 63, 3473.
[12] D. Lelievre, et al. (1995) Tetrahedron Lett., 36, 9317.
[13] P. Hoogerhout, et al. (1999) J. Peptide Res., 54, 436.
[14] P.J. Conolly, et al. (2000) Tetrahedron Lett., 41, 5187.
[15] K. Augustyns, et al. (1998) J. Peptide Res., 51, 127.
[16] A. Srinivasan, et al., Poster 111 presented at the 15th American Peptide Symposium, Nashville, 1997.
[17] S. Peluso, et al. (1999) J. Org. Chem., 64, 7114.
[18] J. J. Diaz-Mochón, et al. (2004) Org. Lett., 6, 1127.
Linkage
Analysis Note
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.5 % (a/a)
Purity (TLC(157B)): ≥ 98 %
Purity (TLC(CMA2)): ≥ 98 %
Assay (HPLC, area%): ≥ 97.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Assay (acidimetric): ≥ 90.0 %
Water (K. F.): ≤ 1.0 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Novabiochem® product range has one of the largest collections of orthogonally and quasi-orthogonally protected tri-functional amino acids. These derivatives are useful tools for the synthesis of cyclic and branched peptides and peptides carrying side-chain modifications.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service