17-10195
ProteoExtract® Cytoskeleton Enrichment and Isolation Kit
This kit provides cytoskeleton purification detergent buffers, sufficient for extraction from ten 100 mm culture dishes, that retain focal adhesion & actin-associated proteins while removing soluble cytoplasmic & nuclear proteins from the cell.
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12161503
eCl@ss:
32161000
Recommended Products
manufacturer/tradename
Chemicon®
ProteoExtract®
technique(s)
western blot: suitable
shipped in
dry ice
General description
Read our application note in Nature Methods!
http://www.nature.com/app_notes/nmeth/2012/121007/pdf/an8624.pdf
(Click Here!)
Rediscovering the Actin Cytoskeleton: New techniques leading to discoveries in cell migration, Dr. Richard Klemke, Morris Cancer Center, UCSD, USA.
The actin cytoskeleton is a highly dynamic network composed of actin polymers and a large variety of associated proteins. The functions of the actin cytoskeleton is to mediate a variety of essential biological functions in all eukaryotic cells, including intra- and extra-cellular movement and structural
Support (Chen, C.S., et al., 2003; Frixione E., 2000). To perform these functions, the organization of the actin cytoskeleton must be tightly regulated both temporally and spatially. Many proteins associated with the actin cytoskeleton are thus likely targets of signaling pathways controlling actin assembly. Actin cytoskeleton assembly is regulated at multiple levels, including the organization of actin monomers (G-actin) into actin polymers and the superorganization of actin polymers into a filamentous network (F-actin – the major constituent of microfilaments) (Bretscher, A., et al., 1994). This superorganization of actin polymers into a filamentous network is mediated by actin side-binding or cross-linking proteins (Dubreuil, R. R., 1991; Matsudaira, P., 1991; Matsudaira, P., 1994). The actin cytoskeleton is a dynamic structure that rapidly changes shape and organization in response to stimuli and cell cycle progression. Therefore, a disruption of its normal regulation may lead to cell transformations and cancer. Transformed cells have been shown to contain less F-actin than untransformed cells and exhibit atypical coordination of F-actin levels throughout the cell cycle (Rao, J.Y., et al., 1990). Orientational distribution of actin filaments within a cell is, therefore, an important determinant of cellular shape and motility.
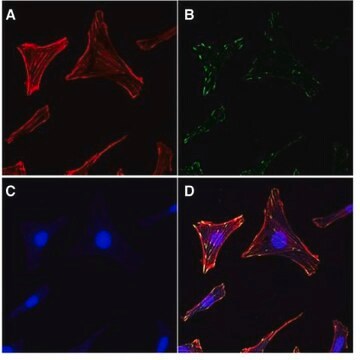
Focal adhesion and adherens junctions are membrane-associated complexes that serve as nucleation sites for actin filaments and as cross-linkers between the cell exterior, plasma membrane and actin cytoskeleton (Yamada, K.M., Geiger, B., 1997). The function of focal adhesions is structural, linking the ECM on the outside to the actin cytoskeleton on the inside. They are also sites of signal transduction, initiating signaling pathways in response to adhesion. Focal adhesions consist of integrin-type receptors that are attached to the extracellular matrix and are intracellularly associated with protein complexes containing vinculin (universal focal adhesion marker), talin, α-actinin, paxillin, tensin, zyxin and focal adhesion kinase (FAK) (Burridge, K., et al., 1990; Turner, C.E., Burridge, K., 1991).
Studying the proteins that associate with and regulate the actin cytoskeleton has been traditionally difficult because of the insolubility of the cytoskeleton in traditional detergents like Triton-X100. Work over the years has shown that many actin regulatory proteins/phospho-proteins upon activation move from the soluble cytoplasmic compartment to the insoluble actin cytoskeleton. The insolubility of these important proteins has made it difficult to study their biochemical changes, such as phosphorylation and nitrosylation, upon binding to actin. What is sorely needed is a cytoskeleton purification method that allows for the selective enrichment of cytoskeleton-associated proteins for detailed protein biochemical analyses. Such a method would provide the means to directly study this important pool of proteins in normal and diseased cytoskeletons.
http://www.nature.com/app_notes/nmeth/2012/121007/pdf/an8624.pdf
(Click Here!)
Rediscovering the Actin Cytoskeleton: New techniques leading to discoveries in cell migration, Dr. Richard Klemke, Morris Cancer Center, UCSD, USA.
The actin cytoskeleton is a highly dynamic network composed of actin polymers and a large variety of associated proteins. The functions of the actin cytoskeleton is to mediate a variety of essential biological functions in all eukaryotic cells, including intra- and extra-cellular movement and structural
Support (Chen, C.S., et al., 2003; Frixione E., 2000). To perform these functions, the organization of the actin cytoskeleton must be tightly regulated both temporally and spatially. Many proteins associated with the actin cytoskeleton are thus likely targets of signaling pathways controlling actin assembly. Actin cytoskeleton assembly is regulated at multiple levels, including the organization of actin monomers (G-actin) into actin polymers and the superorganization of actin polymers into a filamentous network (F-actin – the major constituent of microfilaments) (Bretscher, A., et al., 1994). This superorganization of actin polymers into a filamentous network is mediated by actin side-binding or cross-linking proteins (Dubreuil, R. R., 1991; Matsudaira, P., 1991; Matsudaira, P., 1994). The actin cytoskeleton is a dynamic structure that rapidly changes shape and organization in response to stimuli and cell cycle progression. Therefore, a disruption of its normal regulation may lead to cell transformations and cancer. Transformed cells have been shown to contain less F-actin than untransformed cells and exhibit atypical coordination of F-actin levels throughout the cell cycle (Rao, J.Y., et al., 1990). Orientational distribution of actin filaments within a cell is, therefore, an important determinant of cellular shape and motility.
Focal adhesion and adherens junctions are membrane-associated complexes that serve as nucleation sites for actin filaments and as cross-linkers between the cell exterior, plasma membrane and actin cytoskeleton (Yamada, K.M., Geiger, B., 1997). The function of focal adhesions is structural, linking the ECM on the outside to the actin cytoskeleton on the inside. They are also sites of signal transduction, initiating signaling pathways in response to adhesion. Focal adhesions consist of integrin-type receptors that are attached to the extracellular matrix and are intracellularly associated with protein complexes containing vinculin (universal focal adhesion marker), talin, α-actinin, paxillin, tensin, zyxin and focal adhesion kinase (FAK) (Burridge, K., et al., 1990; Turner, C.E., Burridge, K., 1991).
Studying the proteins that associate with and regulate the actin cytoskeleton has been traditionally difficult because of the insolubility of the cytoskeleton in traditional detergents like Triton-X100. Work over the years has shown that many actin regulatory proteins/phospho-proteins upon activation move from the soluble cytoplasmic compartment to the insoluble actin cytoskeleton. The insolubility of these important proteins has made it difficult to study their biochemical changes, such as phosphorylation and nitrosylation, upon binding to actin. What is sorely needed is a cytoskeleton purification method that allows for the selective enrichment of cytoskeleton-associated proteins for detailed protein biochemical analyses. Such a method would provide the means to directly study this important pool of proteins in normal and diseased cytoskeletons.
Application
Research Category
Cell Structure
Cell Structure
Research Sub Category
Cytoskeleton
Cytoskeleton
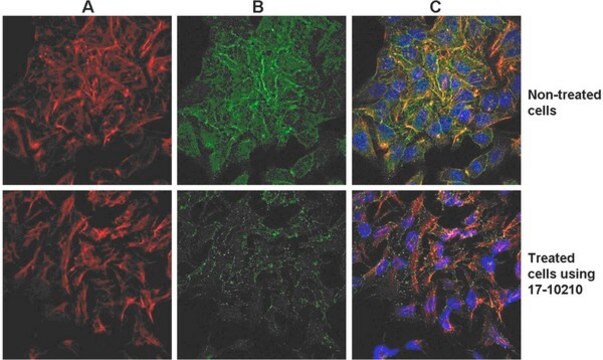
The EMD Millipore ProteoExtract® Cytoskeleton Enrichment and Isolation Kit provides cytoskeleton purification detergent buffers, sufficient for extraction from ten 100 mm culture dishes, that retain focal adhesion and actin-associated proteins while removing soluble cytoplasmic and nuclear proteins from the cell. Anti-Vimentin and anti-GAPDH antibodies are provided as markers for cytoskeleton and cytosol, respectively, in western blot analysis. This kit will greatly increase the ability to detect and study the low abundance actin-associated proteins which are typically masked in conventional methods of Western blotting analysis of whole cell lysates.
This kit provides cytoskeleton purification detergent buffers, sufficient for extraction from ten 100 mm culture dishes, that retain focal adhesion & actin-associated proteins while removing soluble cytoplasmic & nuclear proteins from the cell.
Packaging
Enough Reagents for 15 Reactions/Isolations
Components
One bottle containing 1.9 mL of 10x Cellular Extraction Buffer
One bottle containing 3.5 mL of 20x Cytoskeleton Wash Buffer
One bottle containing 9 mL of ready to use Nuclear Extraction Buffer
Two vials each containing 100 μL of 1000x Protease Inhibitor Cocktail solution
One vial containing 150 μL of 1000x Sodium Orthovanadate solution
One bottle containing 3.8 mL of ready to use Cytoskeleton Solubilization Buffer
One vial containing 30 µL of 2000X goat anti-mouse HRP conjugate
One vial containing 50 µL of 1000x anti-GAPDH mouse monoclonal IgG1 antibody
One vial containing 100 µL of 500x anti-Vimentin mouse monoclonal IgG1 antibody
One bottle containing 3.5 mL of 20x Cytoskeleton Wash Buffer
One bottle containing 9 mL of ready to use Nuclear Extraction Buffer
Two vials each containing 100 μL of 1000x Protease Inhibitor Cocktail solution
One vial containing 150 μL of 1000x Sodium Orthovanadate solution
One bottle containing 3.8 mL of ready to use Cytoskeleton Solubilization Buffer
One vial containing 30 µL of 2000X goat anti-mouse HRP conjugate
One vial containing 50 µL of 1000x anti-GAPDH mouse monoclonal IgG1 antibody
One vial containing 100 µL of 500x anti-Vimentin mouse monoclonal IgG1 antibody
Storage and Stability
Maintain the unopened kit modules (17-10195-1 & 17-10195-2) at the indicated temperatures. The kit may be used for up to 4 months after receipt. Avoid repeated freeze/thaw cycles, and aliquot if necessary.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
PROTEOEXTRACT is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
10 - Combustible liquids
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Viorica L Lastun et al.
The Journal of biological chemistry, 298(6), 101935-101935 (2022-04-19)
In metazoans, the architecture of the endoplasmic reticulum (ER) differs between cell types and undergoes major changes throughout the cell cycle and according to physiological needs. Although much is known about how the different ER morphologies are generated and maintained
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service