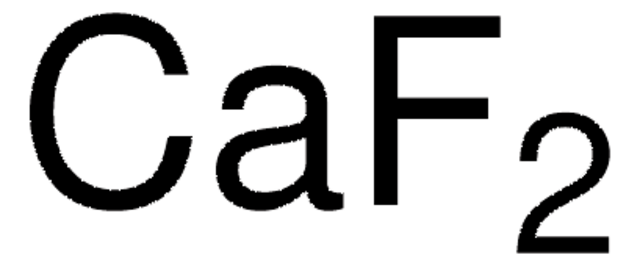1.02840
Calcium fluoride
precipitated pure, Technipur®
Synonym(s):
Calcium difluoride, Fluorite, Fluorspar
About This Item
Recommended Products
Quality Level
pH
4 (20 °C, 100 g/L in H2O, slurry)
bp
2500 °C (lit.)
mp
1418 °C
density
3.18 g/mL at 25 °C (lit.)
SMILES string
F[Ca]F
InChI
1S/Ca.2FH/h;2*1H/q+2;;/p-2
InChI key
WUKWITHWXAAZEY-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Application
In addition, CaF2 is commonly used as a laser crystal and it is easy to grow in the form of large, good-quality bulk crystals to develop commercial optics. It is also used in ceramic host laser applications because of its cubic structure. Divalent rare-earth ions (Sm+2) and trivalent rare-earth ions (Er+3, Tm+3, and Yb+3) are doped with CaF2 to develop a single crystal for tunable broadband and high-power diode-pumped laser operations.
Legal Information
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service