1.00635
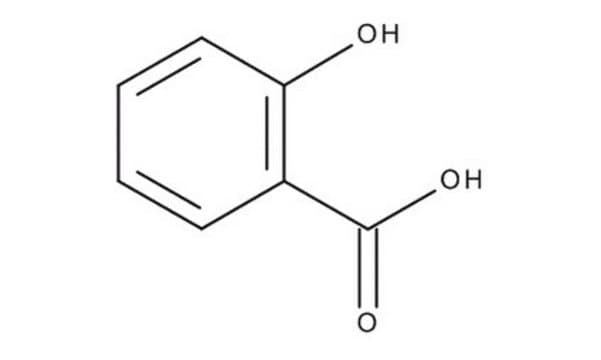
Salicylic acid
fine powder EMPROVE® ESSENTIAL Ph Eur,BP,USP
Pharma Manufacturing
Synonym(s):
Salicylic acid, 2-Hydroxybenzoic acid
About This Item
Recommended Products
Agency
BP
Ph. Eur.
USP
Quality Level
vapor pressure
<1 hPa ( 100 °C)
product line
EMPROVE® ESSENTIAL
Assay
98.0-102.0% dry basis (HPLC)
form
solid
autoignition temp.
500 °C
potency
1250-1580 mg/kg LD50, oral (Rat)
>2000 mg/kg LD50, skin (Rat)
technique(s)
tissue culture: suitable
pH
2.4 ( in H2O, saturated solution)
bp
211 °C/1013 hPa
mp
157-159 °C
transition temp
flash point 157 °C
solubility
water: 2 g/L at 20 °C
density
1.443 g/cm3 at 20 °C
bulk density
400‑500 kg/m3
anion traces
chloride (Cl-): ≤0.0100%
sulfate (SO42-): ≤0.0200%
application(s)
liquid formulation
pharmaceutical
storage temp.
15-25°C
InChI
1S/C7H6O3/c8-6-4-2-1-3-5(6)7(9)10/h1-4,8H,(H,9,10)
InChI key
YGSDEFSMJLZEOE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
314.6 °F - closed cup
Flash Point(C)
157 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Summary application report for analysis of moisture in Amino acids
Summary application report for analysis of moisture in 2-Aminoethanol, Ethanolamin
Summary application report for analysis of moisture in Triethylamine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service