700022P
Avanti
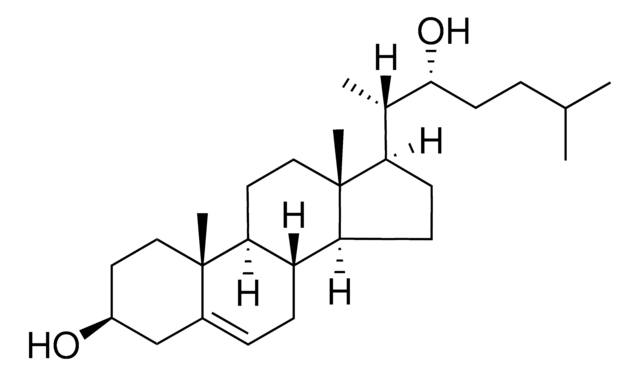
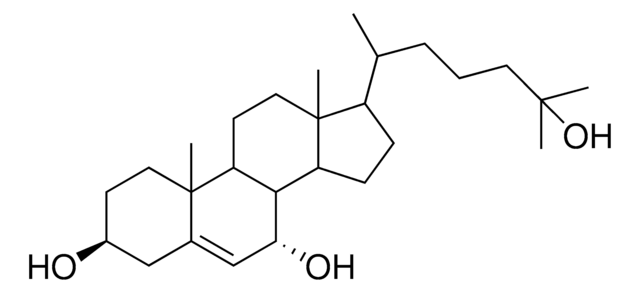
7-keto-27-hydroxycholesterol
Avanti Research™ - A Croda Brand
Synonym(s):
3β,27-dihydroxy-5-cholesten-7-one
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C27H44O3
CAS Number:
Molecular Weight:
416.64
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 1 mg (700022P-1mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand
shipped in
dry ice
storage temp.
−20°C
General description
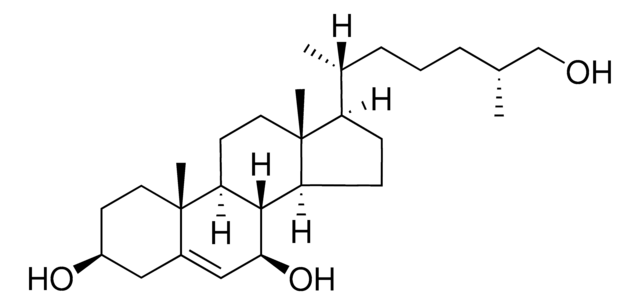
7-keto-27-hydroxycholesterol interconversion to 7β,(25R)26-dihydroxycholesterol (7β,26-diHC) is catalyzed by hydroxysteroid 11-β dehydrogenase. It is also formed by the oxidation of 7-oxocholesterol (7-OC) in the presence of enzyme by sterol 26-hydroxylase (CYP27A1).
Application
7-keto-27-hydroxycholesterol has been used as an oxysterol compound to study its interaction with smoothened (SMO) protein, as a substrate to 11β-hydroxysteroid dehydrogenases (11β-HSDs) for kinetic measurement studies,
Biochem/physiol Actions
7-keto-27-hydroxycholesterol (7-OC) acts as an agonist for the smoothened (SMO) protein of Hedgehog (Hh) signalling pathway, which is vital for proper cell differentiation in embryonic tissue. It elicits strong affinity to SMO compared to 7β,(25R)26-dihydroxycholesterol (7β,26-diHC). 7-OC is reduced to 7β,(25R)26-dihydroxycholesterol (7β,26-diHC) by reactive oxygen species (ROS).
Packaging
5 mL Amber Glass Screw Cap Vial (700022P-1mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Katharina R Beck et al.
Journal of lipid research, 60(9), 1535-1546 (2019-07-06)
Oxysterols previously were considered intermediates of bile acid and steroid hormone biosynthetic pathways. However, recent research has emphasized the roles of oxysterols in essential physiologic processes and in various diseases. Despite these discoveries, the metabolic pathways leading to the different
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service