T30406
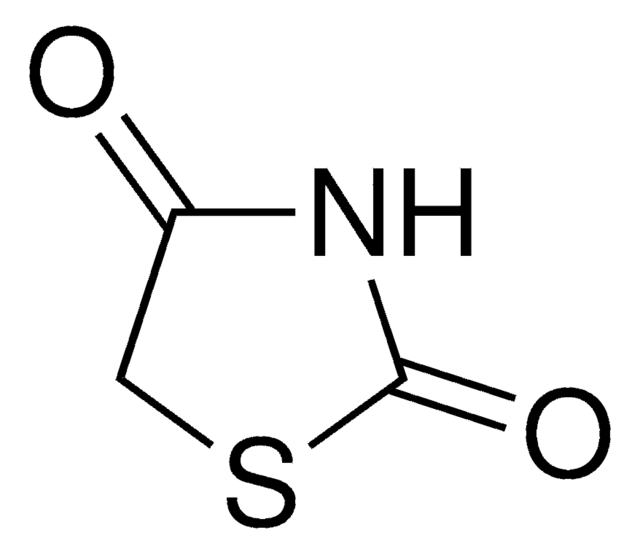
2-Thiohydantoin
99%
Synonym(s):
2-Thioxo-4-imidazolidinone, NSC 11772
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H4N2OS
CAS Number:
Molecular Weight:
116.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
4 (vs air)
Quality Level
Assay
99%
mp
229-231 °C (dec.) (lit.)
SMILES string
O=C1CNC(=S)N1
InChI
1S/C3H4N2OS/c6-2-1-4-3(7)5-2/h1H2,(H2,4,5,6,7)
InChI key
UGWULZWUXSCWPX-UHFFFAOYSA-N
Application
Reactant for synthesis of:
Reactant for persilylation
- Drugs with antidiabetic activity
- Barbituric acid and thiohydantoin derivatives with antimicrobial activity
- Possible anticancer agents
- Fibroblast growth factor receptor 1 kinase inhibitors
- HIV-1 integrase inhibitors
Reactant for persilylation
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Shruti Beharry et al.
Journal of ethnopharmacology, 211, 126-170 (2017-08-16)
Maca - Lepidium meyenii Walp. has been cultivated and used by Andean people for over 1300-2000 years in Peru as food and medicine. Starting in the late 1990's it has developed into an important herbal medicine in China and is
Klaus Abraham et al.
Archives of toxicology, 93(2), 331-340 (2018-12-12)
Fatty acid esters of glycidol (glycidyl esters) are heat-induced food contaminants predominantly formed during industrial deodorization of vegetable oils and fats. After consumption, the esters are digested in the gastrointestinal tract, leading to a systemic exposure to the reactive epoxide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service