M68423
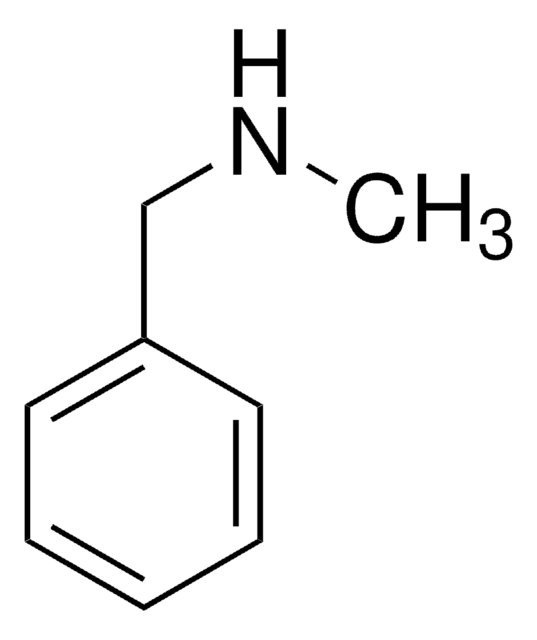
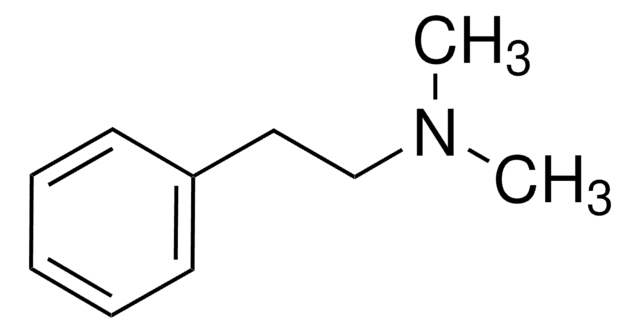
N-Methyl-phenethylamine
99%
Synonym(s):
N-Methyl-2-phenylethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2CH2NHCH3
CAS Number:
Molecular Weight:
135.21
Beilstein:
636347
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.516 (lit.)
bp
203 °C (lit.)
density
0.93 g/mL at 25 °C (lit.)
SMILES string
CNCCc1ccccc1
InChI
1S/C9H13N/c1-10-8-7-9-5-3-2-4-6-9/h2-6,10H,7-8H2,1H3
InChI key
SASNBVQSOZSTPD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N-Methyl-phenethylamine can be used as a reactant:
- To synthesize N-methyl-phenethylamine based tertiary amines by reacting with different alkyl halides in the presence of triphenylphosphine (TPP) and diisopropylazocarboxylate (DIAD) via N-alkylation reaction.
- To fabricate photochemically stable, super-sensitive, and highly selective fluorescent film for the detection of N-methamphetamine (an illicit drug).
- To prepare biologically active squaric acid N-hydroxylamide amide derivatives.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Efficient synthesis of tertiary amines from secondary amines
Michio K, et al.
Tetrahedron Letters, 47, 4871-4875 (2006)
Fabrication of a new fluorescent film and its superior sensing performance to N-methamphetamine in vapor phase
H Meixia, et al.
Sensors and Actuators B, Chemical, 227 (2016)
T D Forbes et al.
Journal of animal science, 72(2), 464-469 (1994-02-01)
Eighteen Suffolk and Suffolk x Hampshire wethers (56.3 +/- 1.3 kg) were used to determine the effects of naturally occurring amines, N-methyl-beta-phenethylamine (NMP) and tyramine (T), on plasma cortisol, norepinephrine (NE), ACTH, and GnRH-stimulated LH concentrations. In each experiment, wethers
J D Duncan et al.
Drug metabolism and disposition: the biological fate of chemicals, 11(1), 15-20 (1983-01-01)
The effects of methyl, ethyl, isopropyl, isobutyl, and benzyl substituents at the alpha-carbon of N-methyl-2-phenethylamine on the kinetics of its N-demethylation in liver microsomes from both control and phenobarbital pretreated rats were studied. In control microsomes, the kinetic studies indicated
I Osamu
European journal of nuclear medicine, 8(9), 385-388 (1983-01-01)
A new type of metabolically trapped agent for measuring regional brain function was designed and evaluated. N-methylphenylethylamine (14C-MPEA) was synthesized with trifluoroacetylphenylethylamine and 14C-methyl iodide. A high concentration of 14C-MPEA accumulated in mouse brain 1 min after injection, and radioactivities
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service