I3750
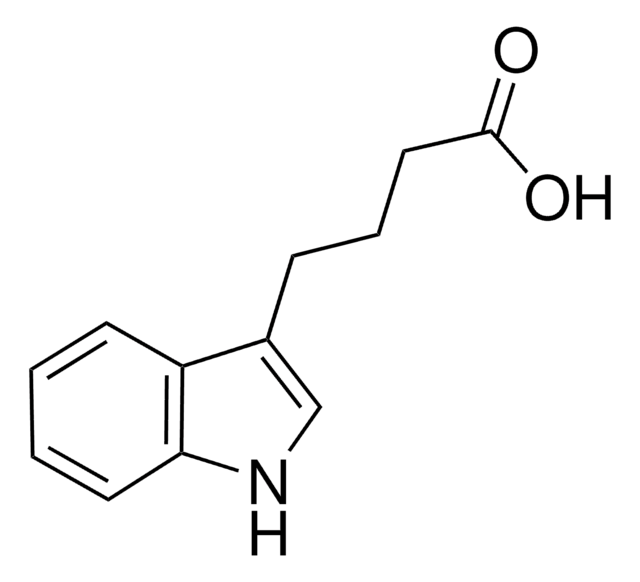
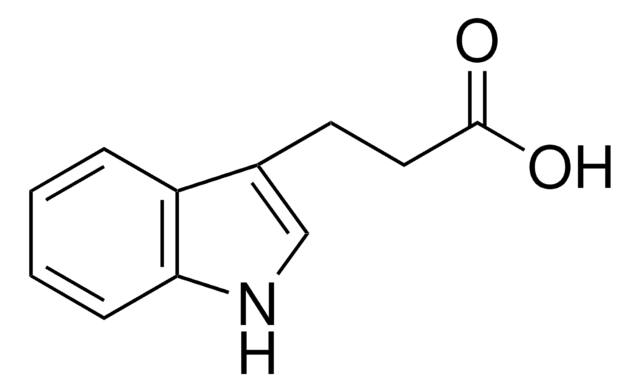
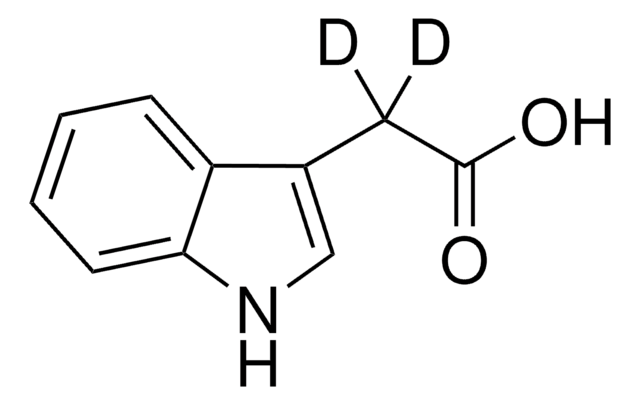
3-Indoleacetic acid
98%
Synonym(s):
Heteroauxin, IAA
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C10H9NO2
CAS Number:
Molecular Weight:
175.18
Beilstein:
143358
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
165-169 °C (lit.)
SMILES string
OC(=O)Cc1c[nH]c2ccccc12
InChI
1S/C10H9NO2/c12-10(13)5-7-6-11-9-4-2-1-3-8(7)9/h1-4,6,11H,5H2,(H,12,13)
InChI key
SEOVTRFCIGRIMH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Indoleacetic acid (IAA) is a naturally occurring hydrophilic organic compound consists of an unsaturated aromatic ring that is commonly used in the synthesis of indolylated diarylmethanes, α-acetoxyl ketones, and Indolmethyl-Chromones via copper catalyzed coupling reactions.
Application
3-Indoleacetic acid (IAA) can be used as a building block to synthesize ,(±) harmacine using 4,4-diethoxybutan-1-amine via an acid-catalyzed acyl iminium ion cyclization reaction.
(±)-Harmacine is a vital step in the synthesis of a variety of indole alkaloids and dopamine/serotonin receptor ligands. IAA can also be used in the preparation of alkaloid (±)-19-hydroxyibogamine.
(±)-Harmacine is a vital step in the synthesis of a variety of indole alkaloids and dopamine/serotonin receptor ligands. IAA can also be used in the preparation of alkaloid (±)-19-hydroxyibogamine.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hyojin Kim et al.
Proceedings of the National Academy of Sciences of the United States of America, 116(15), 7214-7219 (2019-03-30)
Controlling gene expression with sophisticated logic gates has been and remains one of the central aims of synthetic biology. However, conventional implementations of biocomputers use central processing units (CPUs) assembled from multiple protein-based gene switches, limiting the programming flexibility and
Kim L Johnson et al.
PloS one, 10(7), e0131103-e0131103 (2015-07-07)
Mitogen-activated dual-specificity MAPK phosphatases are important negative regulators in the MAPK signalling pathways responsible for many essential processes in plants. In a screen for mutants with reduced organ size we have identified a mutation in the active site of the
L O Villacorte et al.
Water research, 73, 216-230 (2015-02-16)
Algal blooms can seriously affect the operation of water treatment processes including low pressure (micro- and ultra-filtration) and high pressure (nanofiltration and reverse osmosis) membranes mainly due to accumulation of algal-derived organic matter (AOM). In this study, the different components
Mieke de Wit et al.
The New phytologist, 208(1), 198-209 (2015-05-13)
Foliar shade triggers rapid growth of specific structures that facilitate access of the plant to direct sunlight. In leaves of many plant species, this growth response is complex because, although shade triggers the elongation of petioles, it reduces the growth
Célia Jeronimo et al.
Cell reports, 28(5), 1206-1218 (2019-08-01)
Genomic DNA is framed by additional layers of information, referred to as the epigenome. Epigenomic marks such as DNA methylation, histone modifications, and histone variants are concentrated on specific genomic sites, where they can both instruct and reflect gene expression.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service