H36001
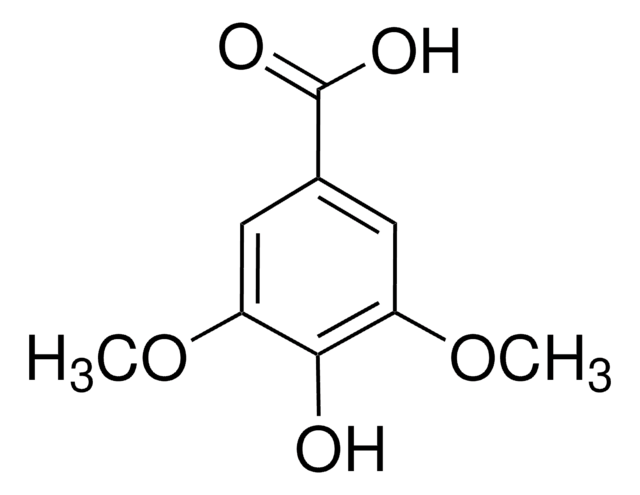
Vanillic acid
97%
Synonym(s):
4-Hydroxy-3-methoxybenzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HOC6H3(OCH3)CO2H
CAS Number:
Molecular Weight:
168.15
Beilstein:
2208364
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
208-210 °C (lit.)
SMILES string
COc1cc(ccc1O)C(O)=O
InChI
1S/C8H8O4/c1-12-7-4-5(8(10)11)2-3-6(7)9/h2-4,9H,1H3,(H,10,11)
InChI key
WKOLLVMJNQIZCI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Vanillic acid, also known as 4-Hydroxy-3-methoxybenzoic acid, is a phenolic molecule, commonly used as a building block in the synthesis of surfactants, flavorants, odorants, and plasticizers.
Application
- Vanillin Synthesis from Vanillic Acid: Research focused on engineering the activity and thermostability of a carboxylic acid reductase for converting vanillic acid to vanillin, providing insights into biotechnological applications for flavor and fragrance industries (Ren et al., 2024).
- Antioxidant Properties in Food Preservation: The antioxidant properties of vanillic acid were evaluated in a study on the preservation of postharvest quality and physicochemical properties of broccoli, suggesting its potential in extending the shelf life and nutritional quality of fresh produce (Kibar et al., 2024).
- Biological Synthesis of Vanillin: A comprehensive review discussed various biological methods for synthesizing vanillin from vanillic acid, emphasizing its application in enhancing natural flavor profiles in the food sector (Venkataraman et al., 2024).
Vanillic acid is used as a starting material in the synthesis of:
- aliphatic-aromatic polymers with good thermal stability and degradability
- novel polyesters via esterification and etherification reaction
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Delphine Lamoral-Theys et al.
Bioorganic & medicinal chemistry, 18(11), 3823-3833 (2010-05-15)
A series of 33 novel divanillates and trivanillates were synthesized and found to possess promising cytostatic rather than cytotoxic properties. Several compounds under study decreased by >50% the activity of Aurora A, B, and C, and WEE1 kinase activity at
B L Lee et al.
Clinical chemistry, 39(9), 1788-1792 (1993-09-01)
We describe a sensitive and specific high-performance liquid-chromatographic method for determining the benzene metabolite, trans,trans-muconic acid (ttMA) in urine by measuring ultraviolet absorbance at 265 nm. We mix 1 mL of urine sample with 2 mL of Tris buffer containing
Changgeng Liu et al.
Environmental science & technology, 46(24), 13262-13269 (2012-11-23)
Methoxyphenols, tracers for wood smoke, are emitted into the atmosphere in large quantities, but their chemical degradation in the atmosphere has not been well characterized. In this study, heterogeneous kinetics of particulate syringaldehyde (SA), vanillic acid (VA), and coniferyl aldehyde
F Mashige et al.
Journal of chromatography. B, Biomedical applications, 658(1), 63-68 (1994-08-05)
An HPLC system for the simultaneous determination of acidic catecholamine metabolites, related compounds and 5-hydroxyindoleacetic acid (5-HIAA) in human urine was developed. A mixed-mode (C18/anion-exchange) column with isocratic elution using citrate buffer and an eight-channel electrochemical detector were used. Vanilmandelic
Zhuliang Tan et al.
Food chemistry, 138(2-3), 1438-1447 (2013-02-16)
Phytosterols and their derivatives have attracted much attention because of their health benefits to humans and are widely used in food, pharmaceuticals, and cosmetics in the past decades. While most of the research has focused on free phytosterols and phytosteryl
Protocols
Coumaric acid; Quercitrin; Myricetin; Quercetin
Global Trade Item Number
| SKU | GTIN |
|---|---|
| H36001-25G | 4061833793541 |
| H36001-100G | 4061833793510 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service