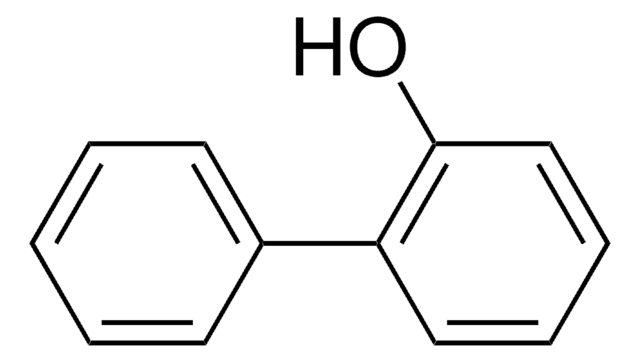
H25808
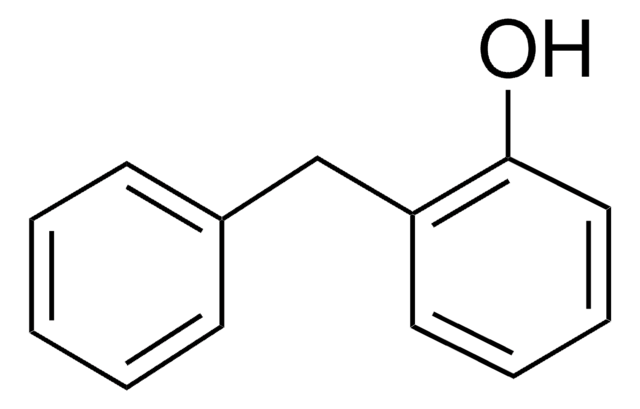
4-Benzylphenol
99%
Synonym(s):
α-Phenyl-p-cresol, 4-Hydroxydiphenylmethane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH2C6H4OH
CAS Number:
Molecular Weight:
184.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
bp
198-200 °C/10 mmHg (lit.)
mp
79-81 °C (lit.)
SMILES string
Oc1ccc(Cc2ccccc2)cc1
InChI
1S/C13H12O/c14-13-8-6-12(7-9-13)10-11-4-2-1-3-5-11/h1-9,14H,10H2
InChI key
HJSPWKGEPDZNLK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hanna Andersson et al.
Bioorganic & medicinal chemistry, 16(14), 6924-6935 (2008-06-17)
Analogues of the hexapeptide angiotensin IV (Ang IV, Val(1)-Tyr(2)-Ile(3)-His(4)-Pro(5)-Phe(6)) encompassing a 4-hydroxydiphenylmethane scaffold replacing Tyr(2) and a phenylacetic or benzoic acid moiety replacing His(4)-Pro(5)-Phe(6) have been synthesized and evaluated in biological assays. The analogues inhibited the proteolytic activity of cystinyl
Kei Takemoto et al.
Mutation research, 519(1-2), 199-204 (2002-08-06)
The genotoxic potential of benzophenone and its metabolically related compounds, benzhydrol and p-benzoylphenol, was investigated using human cytochrome P450 (P450) enzymes. Benzophenone and its two metabolites (0.1-1mM) showed a suppression of bacterial growth without any P450 system, but no induction
A W Stocklinski
Xenobiotica; the fate of foreign compounds in biological systems, 11(6), 425-432 (1981-06-01)
1. Urine and faeces, and two-hour bile samples from adult male rats dosed with [14C]diphenylmethane were analysed for benzhydrol and 2- and 4-hydroxydiphenyl-methane by silica gel GF t.l.c. and 14C-determination. 2. Mean values of 48.4% and 17.7% of the administered
Brandi D Sanders et al.
Bioorganic & medicinal chemistry, 17(19), 7031-7041 (2009-09-08)
The sirtuin proteins are broadly conserved NAD(+)-dependent deacetylases that are implicated in diverse biological processes including DNA recombination and repair, transcriptional silencing, longevity, apoptosis, axonal protection, insulin signaling, and fat mobilization. Because of these associations, the identification of small molecule
Peter J McNamara et al.
Antimicrobial agents and chemotherapy, 53(5), 1898-1906 (2009-02-19)
Menstrual toxic shock syndrome is a rare but potentially life-threatening illness manifest through the actions of Staphylococcus aureus toxic shock syndrome toxin 1 (TSST-1). Previous studies have shown that tampon additives can influence staphylococcal TSST-1 production. We report here on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service