E8260
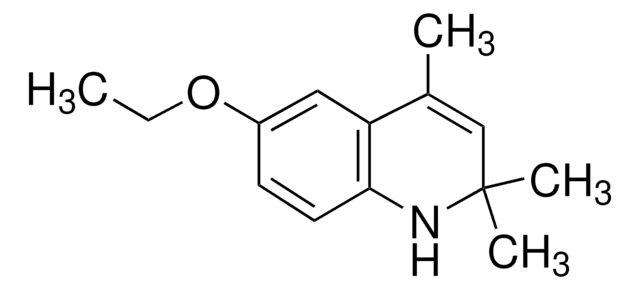
Ethoxyquin
≥75% (capillary GC)
Synonym(s):
1,2-Dihydro-6-ethoxy-2,2,4-trimethylquinoline, 6-Ethoxy-1,2-dihydro-2,2,4-trimethylquinoline
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C14H19NO
CAS Number:
Molecular Weight:
217.31
Beilstein:
158223
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥75% (capillary GC)
form
liquid
density
1.03 g/mL at 20 °C (lit.)
SMILES string
CCOc1ccc2NC(C)(C)C=C(C)c2c1
InChI
1S/C14H19NO/c1-5-16-11-6-7-13-12(8-11)10(2)9-14(3,4)15-13/h6-9,15H,5H2,1-4H3
InChI key
DECIPOUIJURFOJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Alina Błaszczyk et al.
Chemico-biological interactions, 162(1), 70-80 (2006-06-27)
2,2,4,7-Tetramethyl-1,2,3,4-tetrahydroquinoline (THQ) is a new synthetic compound with potential antioxidant activity. In this study, cytotoxic, genotoxic and antioxidant activities of THQ were studied on human lymphocytes with the use of the trypan blue exclusion assay, the TUNEL method, the comet
Rex Munday et al.
Chemico-biological interactions, 155(3), 140-147 (2005-07-28)
Reduction of naphthoquinones by DT-diaphorase is often described as a detoxification reaction. This is true for some naphthoquinone derivatives, such as alkyl and di-alkyl naphthoquinones, but the situation with other substances, such as 2-hydroxy-1,4-naphthoquinone, is more complex. In the present
Alina Błaszczyk et al.
Acta poloniae pharmaceutica, 62(2), 111-115 (2005-09-16)
In our study ethoxyquin (EQ) and its two complexes with flavonoids were obtained from ethoxyquin (1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline, EQ) and quercetin (EQ-Q, 1:1) or rutin (EQ-R, 1:1). Cytotoxicity of the tested compounds was studied using the trypan blue exclusion method and the
Alina Błaszczyk et al.
Cellular & molecular biology letters, 10(1), 15-21 (2005-04-06)
In our study, we analyzed the cytotoxicity of ethoxyquin (EQ) and its two salts, ethoxyquin hydrochloride (EQ-HCL) and ethoxyquin phosphate (EQ-P). It was shown that EQ was the most cytotoxic compound (IC(50) = 0.09 mM), while the lowest cytotoxic effect
Anup G Shah et al.
Water research, 39(17), 4251-4263 (2005-10-04)
The potential inhibitory effect of ethoxyquin, an antioxidant commonly used as a preservative in the food processing industry (e.g., for stabilizing dissolved air flotation residuals), was evaluated at concentrations up to 300 mg/L using a mixed, mesophilic (35 degrees C)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service