E51309
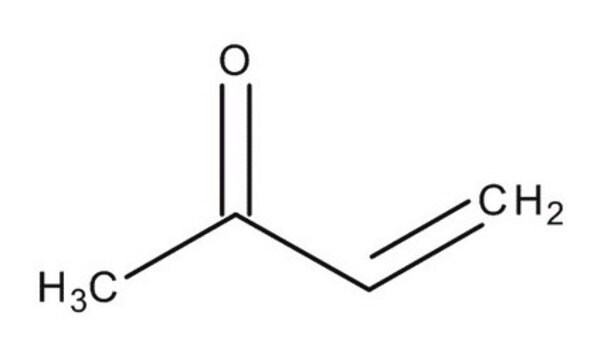
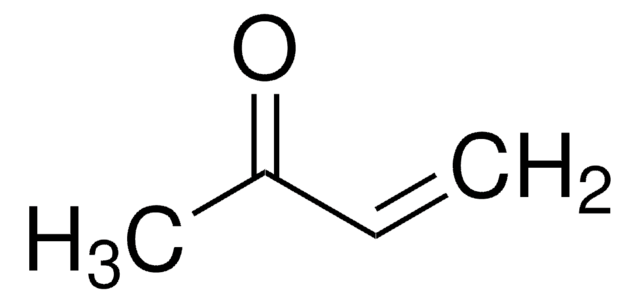
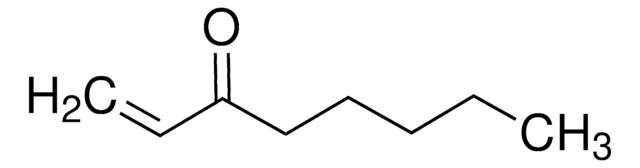
1-Penten-3-one
contains 0.1% BHT as stabilizer, 97%
Synonym(s):
Ethyl vinyl ketone
About This Item
Recommended Products
Quality Level
Assay
97%
form
liquid
contains
0.1% BHT as stabilizer
refractive index
n20/D 1.419 (lit.)
bp
38 °C/60 mmHg (lit.)
density
0.851 g/mL at 20 °C
0.845 g/mL at 25 °C (lit.)
storage temp.
−20°C
SMILES string
CCC(=O)C=C
InChI
1S/C5H8O/c1-3-5(6)4-2/h3H,1,4H2,2H3
InChI key
JLIDVCMBCGBIEY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Conjugated dienes via palladium-catalyzed Heck reaction with vinyl bromides.
- β-Amino carbonyl derivatives via solvent-free aza-Michael addition reaction with aromatic amines in the presence of ionic liquid catalyst.
- α-Exo-methylene group bearing β-amino carbonyl compounds via ion-supported triphenylphosphine catalyzed aza‐Morita‐Baylis-Hillman reaction with N-tosyl arylimines.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
14.0 °F - closed cup
Flash Point(C)
-10 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service