D218200
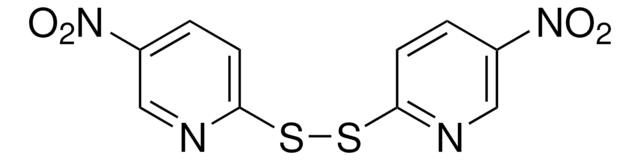
5,5′-Dithiobis(2-nitrobenzoic acid)
ReagentPlus®, 99%
Synonym(s):
3-Carboxy-4-nitrophenyl disulfide, 6,6′-Dinitro-3,3′-dithiodibenzoic acid, Bis(3-carboxy-4-nitrophenyl) disulfide, DTNB, Ellman’s Reagent
About This Item
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
form
powder
mp
240-245 °C (dec.) (lit.)
SMILES string
OC(=O)c1cc(SSc2ccc(c(c2)C(O)=O)[N+]([O-])=O)ccc1[N+]([O-])=O
InChI
1S/C14H8N2O8S2/c17-13(18)9-5-7(1-3-11(9)15(21)22)25-26-8-2-4-12(16(23)24)10(6-8)14(19)20/h1-6H,(H,17,18)(H,19,20)
InChI key
KIUMMUBSPKGMOY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
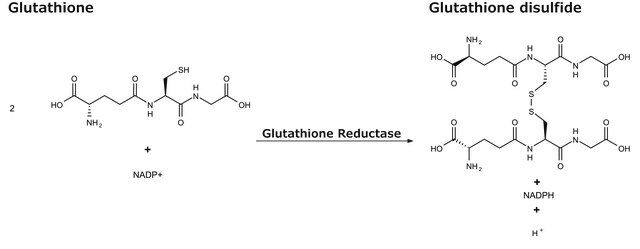
General description
Application
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| D218200-1G | 4061835282197 |
| D218200-5G | 4061835282203 |
| D218200-10G | 4061835282180 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service