D105937
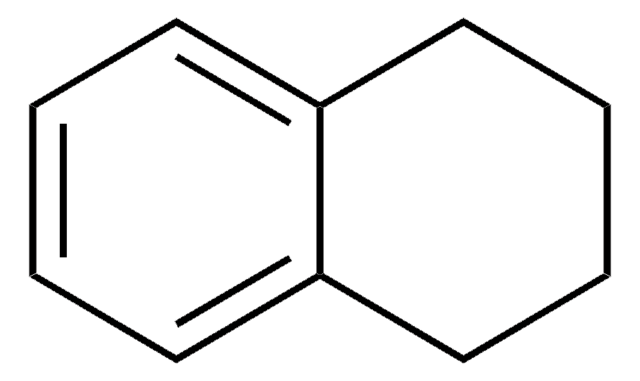
1,2-Dihydronaphthalene
95%
Synonym(s):
1,2-Dialin, 3,4-Dihydronaphthalene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H10
CAS Number:
Molecular Weight:
130.19
Beilstein:
1851372
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥94.5% (GC)
95%
form
liquid
refractive index
n20/D 1.582 (lit.)
bp
89 °C/16 mmHg (lit.)
mp
−8 °C (lit.)
density
0.997 g/mL at 25 °C (lit.)
SMILES string
C1Cc2ccccc2C=C1
InChI
1S/C10H10/c1-2-6-10-8-4-3-7-9(10)5-1/h1-3,5-7H,4,8H2
InChI key
KEIFWROAQVVDBN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
152.6 °F - closed cup
Flash Point(C)
67 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S L Eaton et al.
Applied and environmental microbiology, 62(12), 4388-4394 (1996-12-01)
The substrate oxidation profiles of Sphingomonas yanoikuyae B1 biphenyl-2,3-dioxygenase and cis-biphenyl dihydrodiol dehydrogenase activities were examined with 1,2-dihydronaphthalene and various cis-diols as substrates. m-Xylene-induced cells of strain B1 oxidized 1,2-dihydronaphthalene to (-)-(1R,2S)-cis-1,2-dihydroxy-1,2-3,4-tetrahydronaphthalene as the major product (73% relative yield). Small
Keith Smith et al.
Chemical communications (Cambridge, England), (8)(8), 886-887 (2002-07-19)
We have successfully prepared an unsymmetrical analogue of a Katsuki-type salen ligand having a single hydroxyalkyl group at its 6-position, and also its Mn(III) complex; attachment of the complex to a polymer gives a highly enantioselective and recoverable catalyst for
Tian-Yu Liu et al.
Chemical communications (Cambridge, England), (22)(22), 2228-2230 (2007-05-31)
The asymmetric Michael-type Friedel-Crafts reaction of naphthols and nitroolefins promoted by bifunctional thiourea-tertiary amine organocatalysts (up to 95% ee) was investigated; on simply extending the reaction time further cascade reactions could occur to generate enantiopure dimeric tricyclic 1,2-dihydronaphtho[2,1-b]furanyl-2-hydroxylamine derivatives.
Luiz F Silvia et al.
Molecules (Basel, Switzerland), 10(11), 1419-1428 (2007-11-17)
The oxidation of 2-(3,4-dihydronaphthalen-1-yl)-ethanol (1) with a variety of thallium(III) salts was investigated. An indan, formed by a ring contraction reaction, was obtained in good to moderate yields under a variety of reaction conditions: i) thallium triacetate (TTA) in aqueous
D S Torok et al.
Journal of bacteriology, 177(20), 5799-5805 (1995-10-01)
Bacterial strains expressing toluene and naphthalene dioxygenase were used to examine the sequence of reactions involved in the oxidation of 1,2-dihydronaphthalene. Toluene dioxygenase of Pseudomonas putida F39/D oxidizes 1,2-dihydronaphthalene to (+)-cis-(1S,2R)-dihydroxy-1,2,3,4-tetrahydronaphthalene, (+)-(1R)-hydroxy-1,2-dihydronaphthalene, and (+)-cis-(1R,2S)-dihydroxy-1,2-dihydronaphthalene. In contrast, naphthalene dioxygenase of Pseudomonas
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service