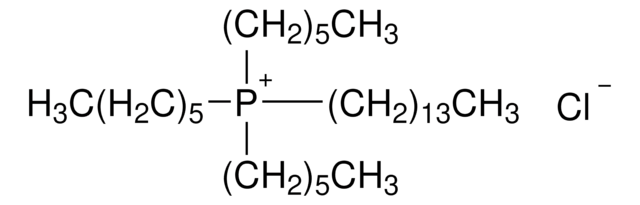96662
Trihexyltetradecylphosphonium bromide
≥95%
Synonym(s):
Tetradecyltrihexylphosphonium bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[CH3(CH2)5]3P(Br)(CH2)13CH3
CAS Number:
Molecular Weight:
563.76
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
≥95.0% (T)
form
solid
density
0.96 g/mL at 20 °C (lit.)
functional group
phosphine
SMILES string
[Br-].CCCCCCCCCCCCCC[P+](CCCCCC)(CCCCCC)CCCCCC
InChI
1S/C32H68P.BrH/c1-5-9-13-17-18-19-20-21-22-23-24-28-32-33(29-25-14-10-6-2,30-26-15-11-7-3)31-27-16-12-8-4;/h5-32H2,1-4H3;1H/q+1;/p-1
InChI key
RJELOMHXBLDMDB-UHFFFAOYSA-M
Application
Reactant for:
- Preparation of ionic liquids via solvent-free anion metathesis reaction
- Preparation of tetraalkylphosphonium tungstophosphate and isopolytungstate Lindquist cluster anion
Trihexyltetradecylphosphonium bromide is a phosphonium-based ionic liquid that can be used as a recyclable reaction medium for Heck cross-coupling reactions. It can also be used to prepare supported liquid membranes (SLMs) for gas separation processes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Heck reactions of aryl halides in phosphonium salt ionic liquids: library screening and applications.
Gerritsma DA, et al.
Tetrahedron Letters, 45(41), 7629-7631 (2004)
Gas separation properties of supported liquid membranes prepared with unconventional ionic liquids.
Cserjesi P, et al.
Journal of Membrane Science, 349(1), 6-11 (2010)
Guokai Cui et al.
Chemistry, an Asian journal, 12(21), 2863-2872 (2017-08-26)
A new strategy involving the computer-assisted design of substituted imidazolate-based ionic liquids (ILs) through tuning the absorption enthalpy as well as the basicity of the ILs to improve SO
Sarah F R Taylor et al.
Physical chemistry chemical physics : PCCP, 19(22), 14306-14318 (2017-05-26)
This study reports on understanding the formation of bubbles in ionic liquids (ILs), with a view to utilising ILs more efficiently in gas capture processes. In particular, the impact of the IL structure on the bubble sizes obtained has been
Guokai Cui et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(14), 5632-5639 (2015-02-18)
A new approach has been developed to improve SO2 sorption by cyano-containing ionic liquids (ILs) through tuning the basicity of ILs and cyano-sulfur interaction. Several kinds of cyano-containing ILs with different basicity were designed, prepared, and used for SO2 capture.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service