924245
Deoxazole
≥95%
Synonym(s):
5,7-Di-tert-butyl-3-phenylbenzo[d]oxazol-3-ium tetrafluoroborate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C21H26BF4NO
CAS Number:
Molecular Weight:
395.24
MDL number:
UNSPSC Code:
12161600
NACRES:
NA.22
Recommended Products
Application
Deoxazole is an N-heterocyclic carbene salt which activates free alcohols for a C-C cross coupling reaction with aryl halides. The MacMillan group developed a mild, robust, and selective metallaphotoredox-based cross-coupling platform for the deoxygenative coupling for free alcohols using Deoxazole, a Ni catalyst, and [Ir(dtbbpy)(ppy)2]PF6.
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Zhe Dong et al.
Nature, 598(7881), 451-456 (2021-09-01)
Metal-catalysed cross-couplings are a mainstay of organic synthesis and are widely used for the formation of C-C bonds, particularly in the production of unsaturated scaffolds1. However, alkyl cross-couplings using native sp3-hybridized functional groups such as alcohols remain relatively underdeveloped2. In particular
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service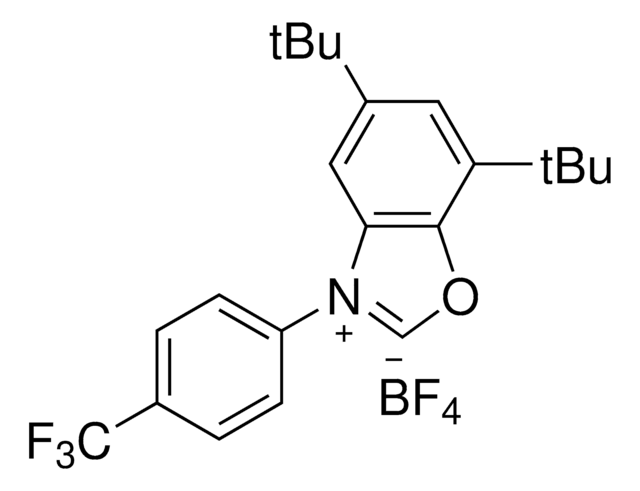
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)
![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)


![[Ir(dF(Me)ppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/150/099/7c2dfa31-39f4-4cca-aee5-86d4a89fea78/640/7c2dfa31-39f4-4cca-aee5-86d4a89fea78.png)
