746738
Lithium hexafluorophosphate solution
in ethylene carbonate and ethyl methyl carbonate, 1.0 M LiPF6 in EC/EMC=50/50 (v/v), battery grade
Synonym(s):
1.0 M LiPF6 EC/EMC=50/50 (v/v), 1.0M LiPF6 EC/MEC=50/50 (v/v), Powerlyte, Purelyte
About This Item
Recommended Products
grade
battery grade
Quality Level
form
solution
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
concentration
(1.0 M LiPF6 in EC/EMC)
impurities
<15 ppm H2O
<50 ppm HF
color
APHA: <50
bp
100 °C
density
1.27 g/mL at 25 °C (lit.)
anion traces
chloride (Cl-): ≤1 ppm
sulfate (SO42-): ≤2 ppm
cation traces
Ca: ≤1 ppm
Fe: ≤1 ppm
K: ≤1 ppm
Na: ≤1 ppm
Pb: ≤1 ppm
application(s)
battery manufacturing
greener alternative category
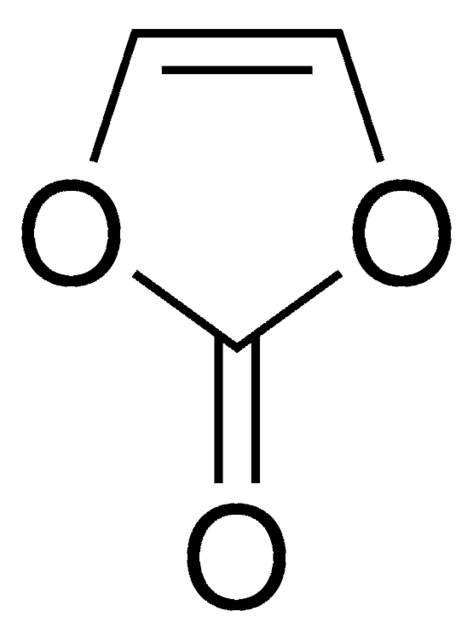
SMILES string
F[P-](F)(F)(F)(F)F.[Li+]
InChI
1S/F6P.Li/c1-7(2,3,4,5)6;/q-1;+1
InChI key
AXPLOJNSKRXQPA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
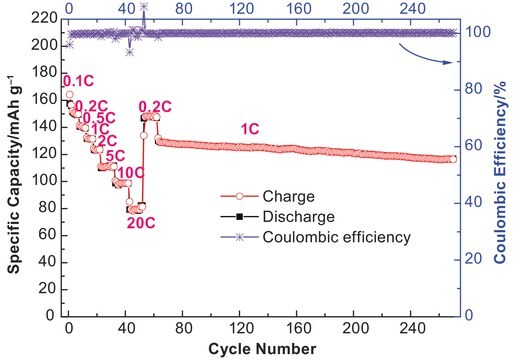
The ready-to-use electrolyte solutions are available in different solvent blends and can support a wide variety of lithium ion battery applications. These solutions are high purity and battery grade thus making them also suitable as standards in LIB research. Customized formulations can be made by inter-mixing the electrolyte solutions or by mixing appropriate of additives.
Other Notes
- Do not use with glass equipment
- All work should be done very quickly under dry air to prevent electrolytes from water uptake and solvent vaporization.
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1A - STOT RE 1 Inhalation - STOT RE 2 Oral
Target Organs
Bone,Teeth, Kidney
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
86.0 °F
Flash Point(C)
30 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Dr. Sun reviews the recent advances in solid-state rechargeable batteries and cover the fundamentals of solid electrolytes in solid-state batteries, the theory of ion conduction, and the structures and electrochemical processes of solid-state Li batteries.
Lithium-ion batteries (LIBs) have been widely adopted as the most promising portable energy source in electronic devices because of their high working voltage, high energy density, and good cyclic performance.
The critical technical challenges associated with the commercialization of electric vehicle batteries include cost, performance, abuse tolerance, and lifespan.
Due to the adverse impact of the continued use of fossil fuels on the earth’s environment and climate, researchers have been asked to develop new approaches for producing power using renewable sources like wind and solar energy
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service