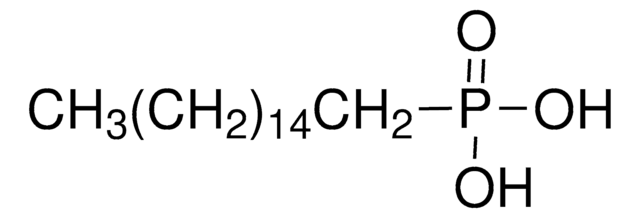735914
Octylphosphonic acid
97%
Synonym(s):
n-Octylphosphonic acid, OPA
About This Item
Recommended Products
Assay
97%
form
solid
mp
93-98 °C
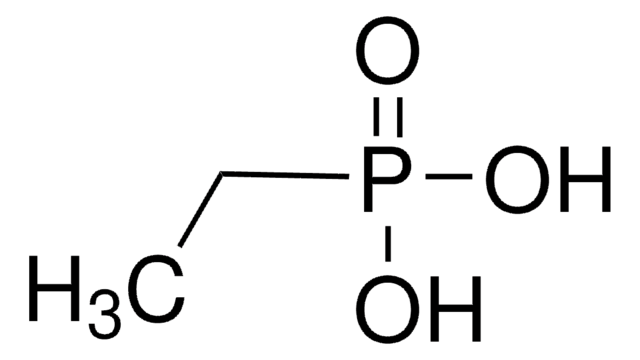
SMILES string
CCCCCCCCP(O)(O)=O
InChI
1S/C8H19O3P/c1-2-3-4-5-6-7-8-12(9,10)11/h2-8H2,1H3,(H2,9,10,11)
InChI key
NJGCRMAPOWGWMW-UHFFFAOYSA-N
General description
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B - STOT RE 2 Oral
Target Organs
Kidney,Bone
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
There is widespread demand for thin, lightweight, and flexible electronic devices such as displays, sensors, actuators, and radio-frequency identification tags (RFIDs). Flexibility is necessary for scalability, portability, and mechanical robustness.
Self-assembled monolayers (SAMs) have attracted enormous interest for a wide variety of applications in micro- and nano-technology. In this article, we compare the benefits of three different classes of SAM systems (alkylthiolates on gold.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service