671479
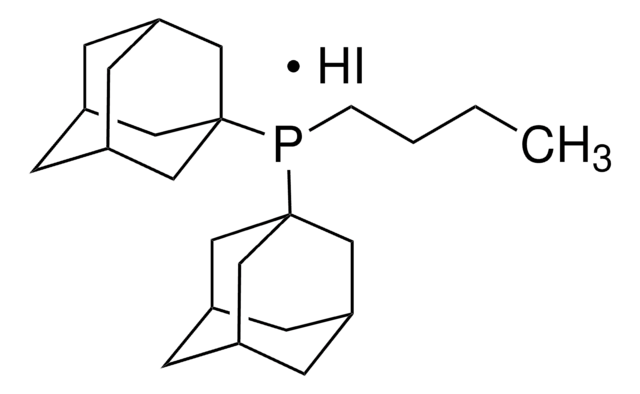
cataCXium® A
95%
Synonym(s):
Di(1-adamantyl)-n-butylphosphine
About This Item
Recommended Products
Quality Level
Assay
95%
reaction suitability
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-C Bond Formation
reagent type: ligand
reaction type: Cross Couplings
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
functional group
phosphine
SMILES string
CCCCP([C@]12C[C@H]3C[C@H](C[C@H](C3)C1)C2)[C@@]45C[C@@H]6C[C@@H](C[C@@H](C6)C4)C5
InChI
1S/C24H39P/c1-2-3-4-25(23-11-17-5-18(12-23)7-19(6-17)13-23)24-14-20-8-21(15-24)10-22(9-20)16-24/h17-22H,2-16H2,1H3/t17-,18+,19-,20-,21+,22-,23-,24-
InChI key
HTJWUNNIRKDDIV-FECFBMJZSA-N
General description
cataCXium® A is commonly used as a catalyst in cross-coupling reactions, such as Suzuki and Sonogashira reactions.
Application
Other applications:
- palladium-catalyzed carbonylation of aryl and heteroaryl halides
- palladium-catalyzed synthesis of (hetero)aromatic nitriles
- palladium-catalyzed aminocarbonylation of aryl halides
Features and Benefits
- Mild reaction condition
- Low catalyst loading
- High yield and turn over number
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
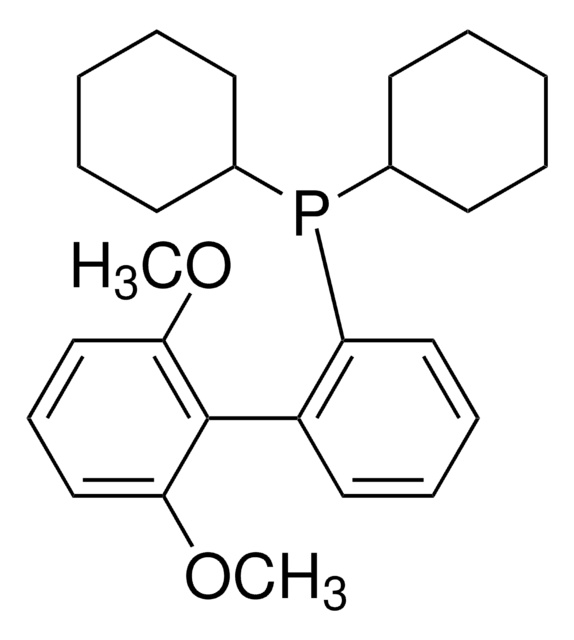
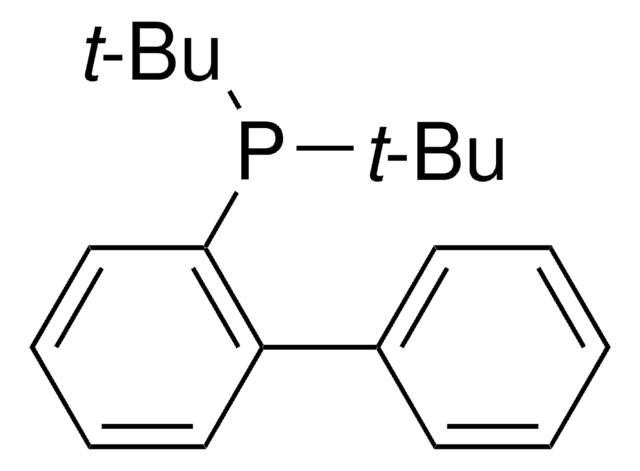
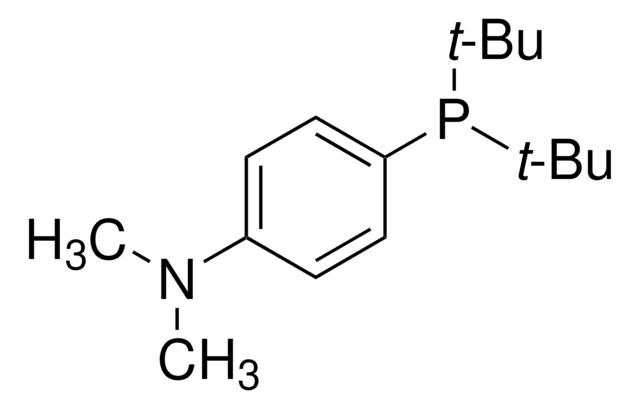
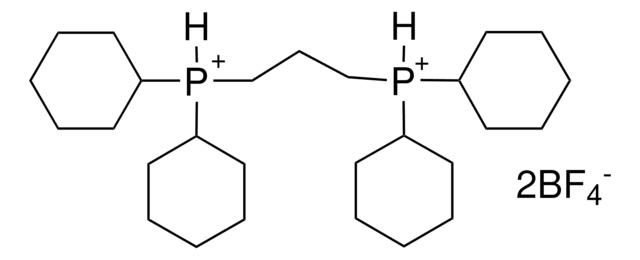
Customers Also Viewed
Articles
cataCXium® - Ligands and Complexes for Efficient Cross-Coupling Reactions. Cross-coupling reactions are an important class of catalytic transformations with applications in polymer science as well as in the fine chemicals and pharmaceutical industries.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service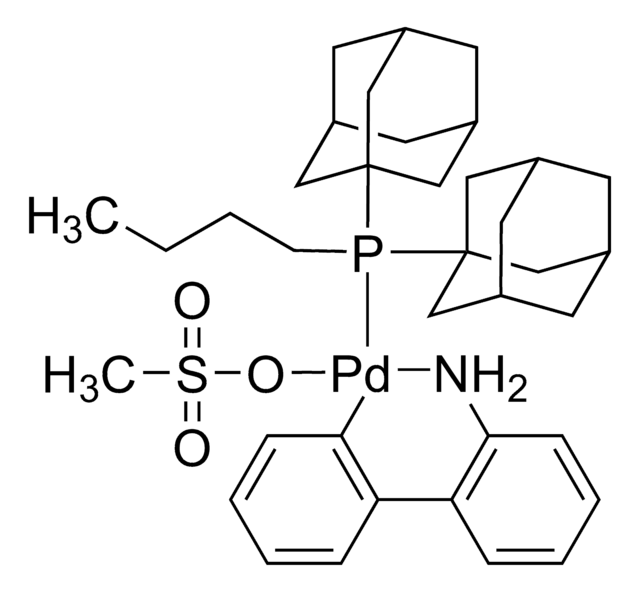




![trans-Bis(acetato)bis[o-(di-o-tolylphosphino)benzyl]dipalladium(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/291/406/8cdb9bda-d2bf-41c5-adfb-c3cc57cf62e8/640/8cdb9bda-d2bf-41c5-adfb-c3cc57cf62e8.png)
![1,4-Diazabicyclo[2.2.2]octane bis(sulfur dioxide) adduct ≥95% (sulfur, elemental analysis)](/deepweb/assets/sigmaaldrich/product/structures/158/739/a9df497b-883d-40f1-ac45-bf699dcee9f9/640/a9df497b-883d-40f1-ac45-bf699dcee9f9.png)