640441
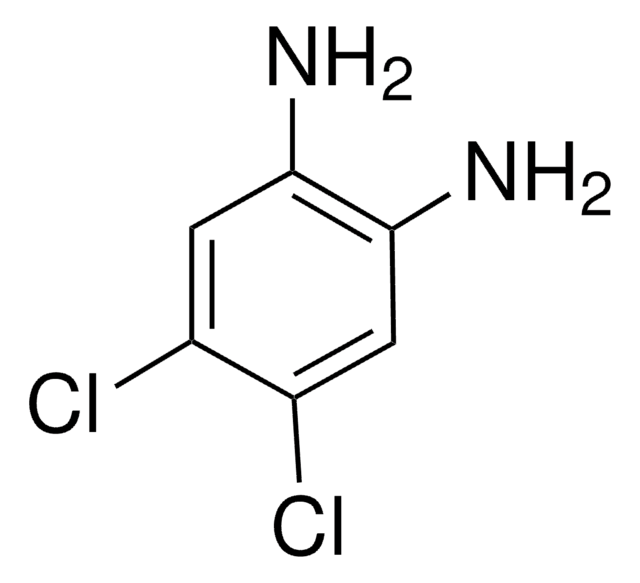
4-Bromo-1,2-diaminobenzene
97%
Synonym(s):
4-Bromo-2-aminoaniline, 4-Bromo-o-phenylenediamine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H7BrN2
CAS Number:
Molecular Weight:
187.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
65-69 °C (dec.) (lit.)
functional group
bromo
SMILES string
Nc1ccc(Br)cc1N
InChI
1S/C6H7BrN2/c7-4-1-2-5(8)6(9)3-4/h1-3H,8-9H2
InChI key
WIHHVKUARKTSBU-UHFFFAOYSA-N
Related Categories
General description
4-Bromo-1,2-diaminobenzene can be obtained from 1,2-diaminobenzene via acetylation followed by bromination and alkaline hydrolysis.
4-Bromo-1,2-diaminobenzene is used as a precursor in the production of tetra-dentate unsymmetrical Schiff base.
4-Bromo-1,2-diaminobenzene is used as a precursor in the production of tetra-dentate unsymmetrical Schiff base.
Application
4-Bromo-1,2-diaminobenzene can be used as a precursor for preparing fluorescent dipolar quinoxaline derivatives, which can find applications as potential emissive and electron-transport materials. It can also be used in the synthesis of 6-bromo-2-methylbenzimidazole.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
"Guanidine chemistry"
Ishikawa T, et al.
Chemical & Pharmaceutical Bulletin, 58(12), 1555-1564 (2010)
"The synthesis and characterization of novel dipolar fluorescent materials based on a quinoxaline core"
Wang H, et al.
Dyes and Pigments, 83(03), 269-275 (2009)
"A PdII Complex Bearing a Benzimidazole-Derived Ligand with Potentially ?Mesoionic and Remote? Character and Its Catalytic Activity"
Huynh VH, et al.
European Journal of Organic Chemistry, 2013(26), 4654-4661 (2013)
Erin Wachter et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 22(2), 550-559 (2015-11-13)
Recognition and regulation of G-quadruplex nucleic acid structures is an important goal for the development of chemical tools and medicinal agents. The addition of a bromo-substituent to the dipyridylphenazine (dppz) ligands in the photophysical "light switch", [Ru(bpy)2 dppz](2+) , and
Jacek E Nycz et al.
Molecules (Basel, Switzerland), 24(22) (2019-11-27)
New approaches to the synthesis of 4,7-dichloro-1,10-phenanthrolines and their corresponding 9H-carbazol-9-yl-, 10H-phenothiazin-10-yl- and pyrrolidin-1-yl derivatives were developed. Their properties have been characterized by a combination of several techniques: MS, HRMS, GC-MS, electronic absorption spectroscopy and multinuclear NMR in both solution
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 640441-5G | 4061832728759 |
| 640441-25G | 4061832728742 |
| 640441-100G | 4061832763378 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service