576646
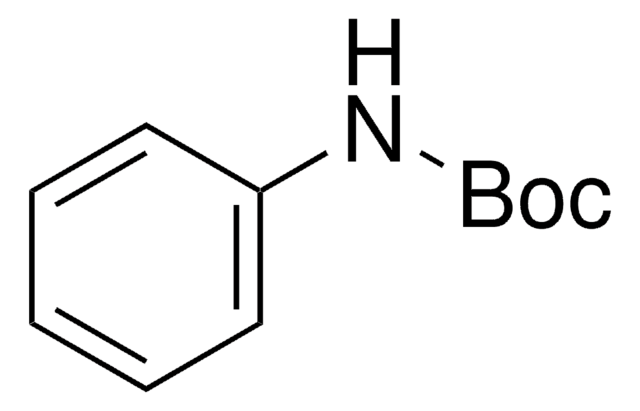
N-Boc-4-hydroxyaniline
97%
Synonym(s):
N-Boc-4-aminophenol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOC6H4NHCO2C(CH3)3
CAS Number:
Molecular Weight:
209.24
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
143-147 °C (lit.)
functional group
amine
SMILES string
CC(C)(C)OC(=O)Nc1ccc(O)cc1
InChI
1S/C11H15NO3/c1-11(2,3)15-10(14)12-8-4-6-9(13)7-5-8/h4-7,13H,1-3H3,(H,12,14)
InChI key
YRQMBQUMJFVZLF-UHFFFAOYSA-N
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Christopher Elam et al.
European journal of medicinal chemistry, 46(5), 1512-1523 (2011-03-01)
Two screening protocols based on recursive partitioning and computational ligand docking methodologies, respectively, were employed for virtual screens of a compound library with 345,000 entries for novel inhibitors of the enzyme sarco/endoplasmic reticulum calcium ATPase (SERCA), a potential target for
Anne Müller et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(39), 13723-13731 (2015-08-08)
Azobenzene linker molecules can be utilized to control peptide/protein function when they are ligated to appropriately spaced amino acid side chains of the peptide. This is because the photochemical E/Z isomerization of the azobenzene N=N double bond allows to switch
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![4-[(N-Boc)aminomethyl]aniline 97%](/deepweb/assets/sigmaaldrich/product/structures/341/155/530c425c-7e6e-435e-a28a-9d40b05b938a/640/530c425c-7e6e-435e-a28a-9d40b05b938a.png)


