537594
tert-Butyl acetoacetate
reagent grade, 98%
Synonym(s):
TBAA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
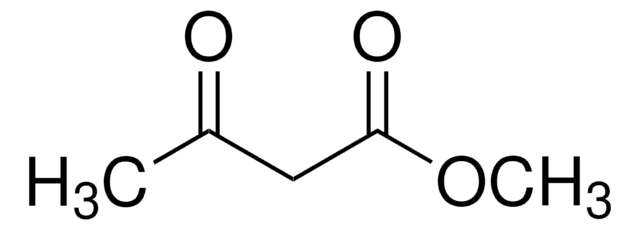
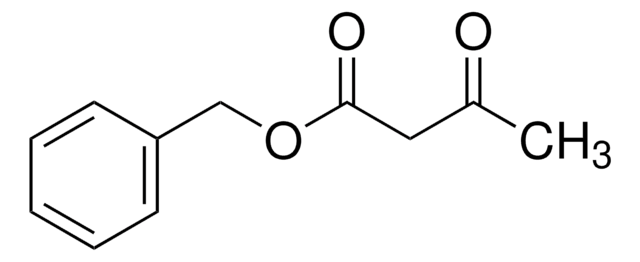
CH3COCH2COOC(CH3)3
CAS Number:
Molecular Weight:
158.19
Beilstein:
1680303
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
reagent grade
Quality Level
Assay
98%
expl. lim.
8 % (lit.)
impurities
≤0.20% water
water
refractive index
n20/D 1.419 (lit.)
bp
71-72 °C/11 mmHg (lit.)
mp
-38 °C (lit.)
density
0.954 g/mL at 25 °C (lit.)
functional group
ester
ketone
SMILES string
CC(=O)CC(=O)OC(C)(C)C
InChI
1S/C8H14O3/c1-6(9)5-7(10)11-8(2,3)4/h5H2,1-4H3
InChI key
JKUYRAMKJLMYLO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
tert-Butyl acetoacetate (t-BAA) is a cheap and easy to store commercial reagent. Reaction demonstrated that the more hindered tert-butyl acetoacetate (t-BAA, la) is ca.15-20-fold more reactive than the more commonly used methyl or ethyl analogs. It is widely employed as an acetoacetylating reagent.
Application
tert-Butyl acetoacetate may be used in the synthesis of:
- various acetoacetic acid derivatives
- acetoacetates
- acetoacetamides
- 1-(diethylamino)-2-acetoacetoxypropane
- (S)-tert-butyl 3-hydroxybutyrate
- benzothiazole β-keto ester derivatives
- 3,4-disubstituted pyrroles
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
150.8 °F - closed cup
Flash Point(C)
66 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of (S)-Tert-Butyl 3-Hydroxybutyrate by Asymmetric Reduction of Tert-Butyl Acetoacetate with Saccharomyces cerevisiae B5.
Ou ZM, et al.
Advanced Materials Research, 704 (2013)
Enzymatic Synergism in the Synthesis of ?-Keto Esters.
Wisniewska C, et al.
European Journal of Organic Chemistry, 24, 5432-5437 (2015)
Highly enantioselective Mannich reactions of imines with tert-butyl acetoacetate catalyzed by squaramide organocatalyst.
He HX and Du DM.
Tetrahedron Asymmetry, 25(8), 637-643 (2014)
Transacetoacetylation with tert-butyl acetoacetate: synthetic applications.
Witzeman JS and Nottingham WD.
The Journal of Organic Chemistry, 56(5), 1713-1718 (1991)
Transesterification.
Otera J.
Chemical Reviews, 93(4), 1449-1470 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service