522929
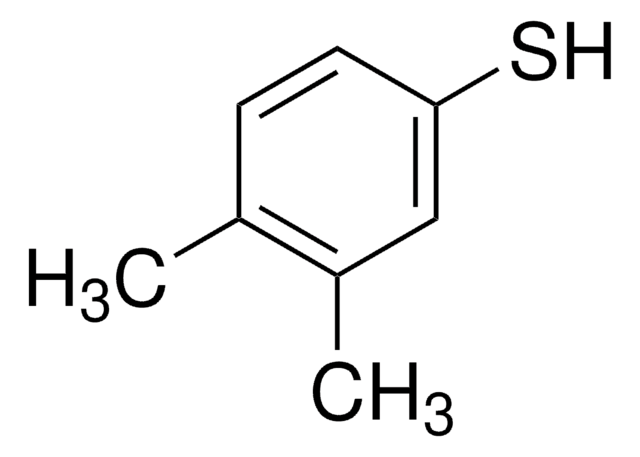
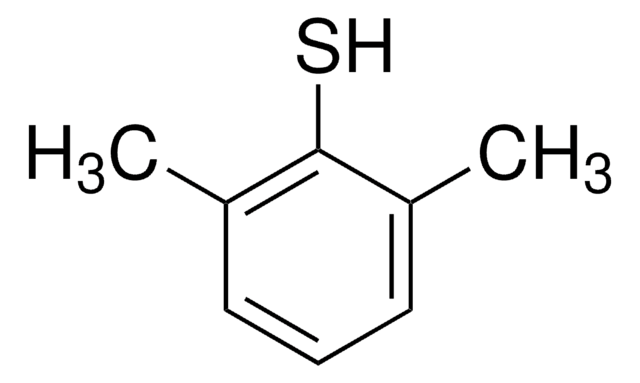
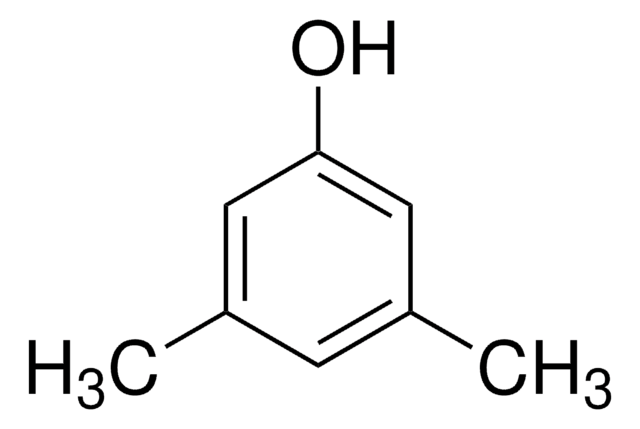
3,5-Dimethylbenzenethiol
90%
Synonym(s):
3,5-Dimethylthiophenol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2C6H3SH
CAS Number:
Molecular Weight:
138.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
90%
refractive index
n20/D 1.568 (lit.)
bp
127.5 °C/50 mmHg (lit.)
density
1.015 g/mL at 25 °C (lit.)
SMILES string
Cc1cc(C)cc(S)c1
InChI
1S/C8H10S/c1-6-3-7(2)5-8(9)4-6/h3-5,9H,1-2H3
InChI key
CESBAYSBPMVAEI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3,5-Dimethylbenzenethiol is an organic building block. It is also referred to as 5-mercapto-m-xylene.
Application
3,5-Dimethylbenzenethiol may be employed as a starting reagent in the preparation of Lithium 2-lithio-3,5-dimethylbenzenethiolate. It may also be used in the preparation of 1,1,2-trichloro-4-(3,5-dimethylphenylthio)-1-buten-3-yne and 1,1,2,4-tetrachloro-4-(3,5-dimethylphenylthio)-1,3-butadiene.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
185.0 °F - closed cup
Flash Point(C)
85 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Directed lithiation of arenethiols.
Smith K, et al.
Journal of the American Chemical Society, 111(2), 665-669 (1989)
Ibis C and Sahin A.
Bull. Korean Chem. Soc., 31(8), 2255-2260 (2010)
Homan Kang et al.
Scientific reports, 5, 10144-10144 (2015-05-29)
Recently, preparation and screening of compound libraries remain one of the most challenging tasks in drug discovery, biomarker detection, and biomolecular profiling processes. So far, several distinct encoding/decoding methods such as chemical encoding, graphical encoding, and optical encoding have been
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service