471496
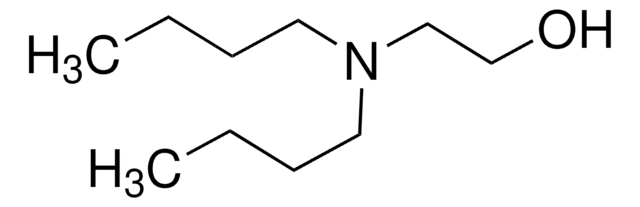
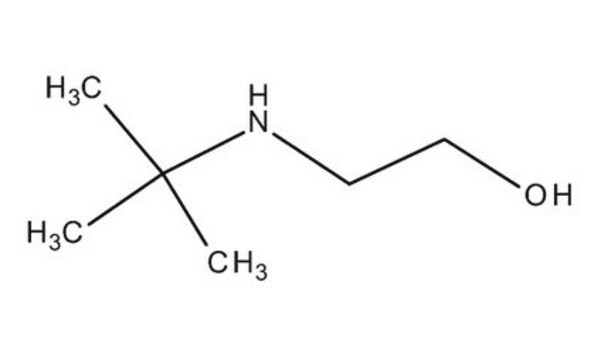
2-(Butylamino)ethanol
≥98%
Synonym(s):
N-Butyl-2-hydroxyethylamine, N-Butylethanolamine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3(CH2)3NHCH2CH2OH
CAS Number:
Molecular Weight:
117.19
Beilstein:
1732522
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
refractive index
n20/D 1.444 (lit.)
bp
198-200 °C (lit.)
density
0.891 g/mL at 25 °C (lit.)
functional group
amine
hydroxyl
SMILES string
CCCCNCCO
InChI
1S/C6H15NO/c1-2-3-4-7-5-6-8/h7-8H,2-6H2,1H3
InChI key
LJDSTRZHPWMDPG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-(Butylamino)ethanol is a secondary amine having a -OH group.
Application
2-(Butylamino)ethanol (N-Butylethanolamine) may be employed for the synthesis of 4-alkylamino-2,5,6-trimethyl -7-(2,4,6-trimethylphenyl)pyrrolo[2,3-d]pyrimidines and N-butyl-N-(2-nitroxyethyl)nitramine (BuNENA).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
185.0 °F
Flash Point(C)
85 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
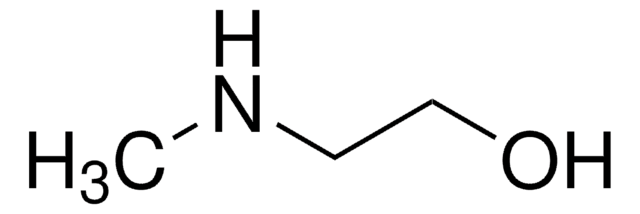
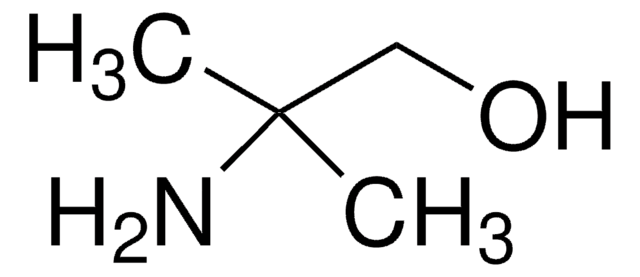
Customers Also Viewed
Sitzmann.
Sitzmann ME, et al.
Propellants, Explosives, Pyrotechnics, 31(2), 124-130 (2006)
M Sandin et al.
Antimicrobial agents and chemotherapy, 34(3), 491-493 (1990-03-01)
The antimicrobial effects of diethanolamine, dimethylamino-methyl-propanol, and butylethanolamine are greatly enhanced at high pH. Their antimicrobial activities are closely correlated with their uncharged forms, indicating that diffusion through cell membrane(s) is rate limiting for the antimicrobial action. Since these compounds
Ling-Wei Hsin et al.
Bioorganic & medicinal chemistry, 10(1), 175-183 (2001-12-12)
A series of compounds related to N-butyl-N-ethyl[2,5,6-trimethyl-7-(2,4,6-trimethylphenyl)pyrrolo[2,3-d]pyrimidin-4-yl]amine (1, antalarmin) have been prepared and evaluated for their CRHR1 binding affinity as the initial step in the development of selective high affinity hydrophilic nonpeptide corticotropin-releasing hormone type 1 receptor (CRHR1) antagonists. Calculated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service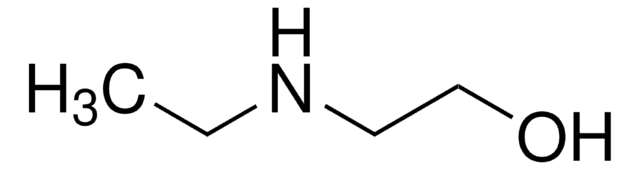





![3-[(2-Aminoethyl)amino]-1-propanol AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/193/734/c3b3e55a-2f61-43c5-8804-ff55209c4b55/640/c3b3e55a-2f61-43c5-8804-ff55209c4b55.png)
