462292
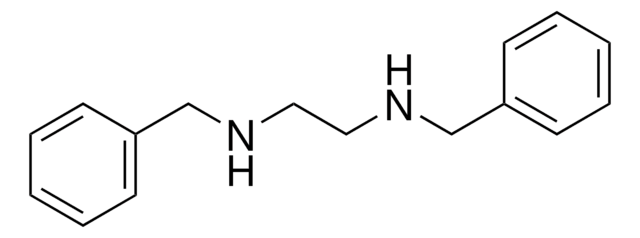
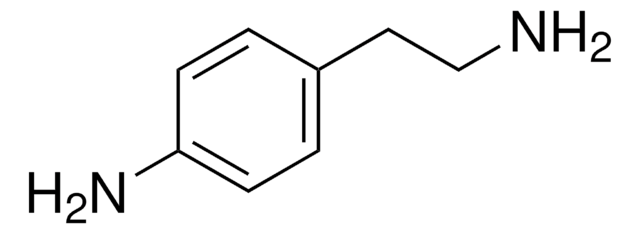
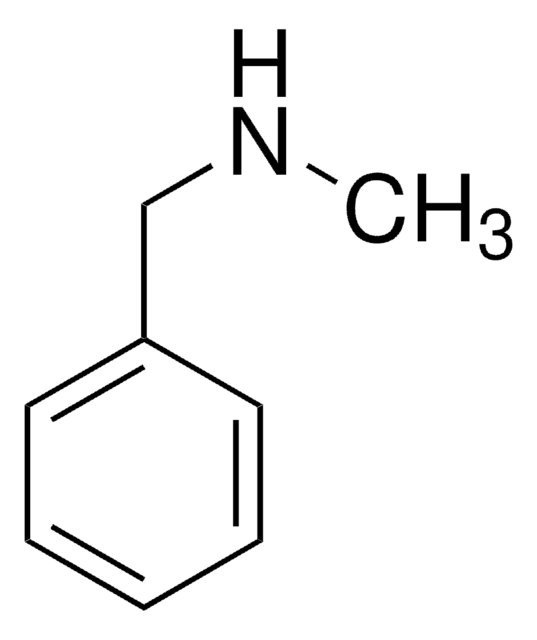
N-Benzylethylenediamine
97%
Synonym(s):
2-Benzylaminoethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
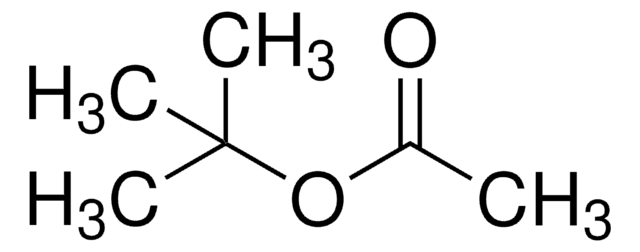
C6H5CH2NHCH2CH2NH2
CAS Number:
Molecular Weight:
150.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.54 (lit.)
bp
162 °C/20 mmHg (lit.)
density
1 g/mL at 25 °C (lit.)
functional group
amine
phenyl
SMILES string
NCCNCc1ccccc1
InChI
1S/C9H14N2/c10-6-7-11-8-9-4-2-1-3-5-9/h1-5,11H,6-8,10H2
InChI key
ACYBVNYNIZTUIL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N-Benzylethylenediamine participates in the one-pot synthesis of N,N,N′-trisubstituted guanidines. It undergoes condensation with dibenzoylmethane (1,3-diphenyl-1,3-propanedione) in stoichiometric ratio 1:1 to afford the corresponding Schiff monobase.
Application
N-Benzylethylenediamine may be used for the synthesis of N-benzyl-N,N′,N′-tris(tert-butyloxycarbonylmethyl)ethylenediamine and 3-benzyl-2-(phenyl-2-sulfonate)-2-imidazoline tetraheptylammonium salt.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
203.0 °F - closed cup
Flash Point(C)
95 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Application of a-chloroaldoxime O-methanesulfonates to one-pot synthesis of N, N', N?-substituted guanidines via Tiemann rearrangement.
Yamamoto Y, et al.
Tetrahedron Letters, 50(42), 5813-5815 (2009)
Anthony Weatherwax et al.
Organic letters, 7(16), 3461-3463 (2005-07-29)
Trans-disubstituted beta-lactams show increasing utility and prominence in numerous pharmaceutical applications, making their asymmetric synthesis an attractive goal for chemists. We introduce an anionic, nucleophilic catalyst system that provides an efficient, diastereoselective route to trans-disubstituted beta-lactams, a complement to our
A new method for the synthesis of tri-tert-butyl diethylenetriaminepentaacetic acid and its derivatives.
Achilefu S, et al.
The Journal of Organic Chemistry, 65(5), 1562-1565 (2000)
Nickel (II) Complexes of Dibenzoylmethane and N-benzylethylenediamine, and Their Schiff Monobase.
Gutierrez JA, et al.
Journal of Coordination Chemistry, 28(3-4), 305-312 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service