453757
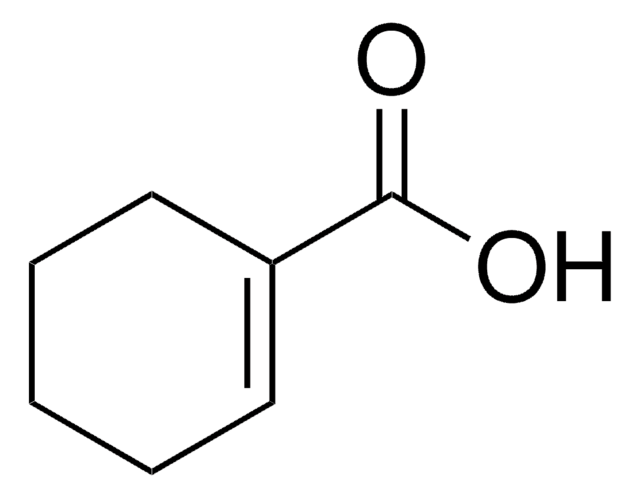
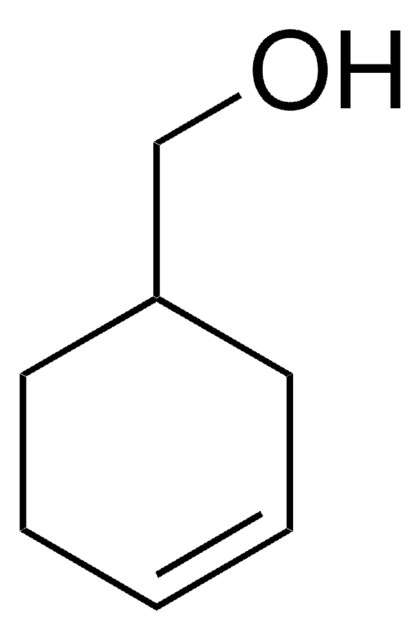
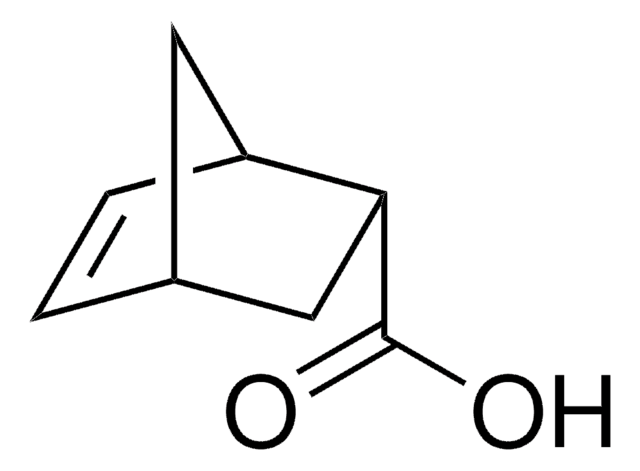
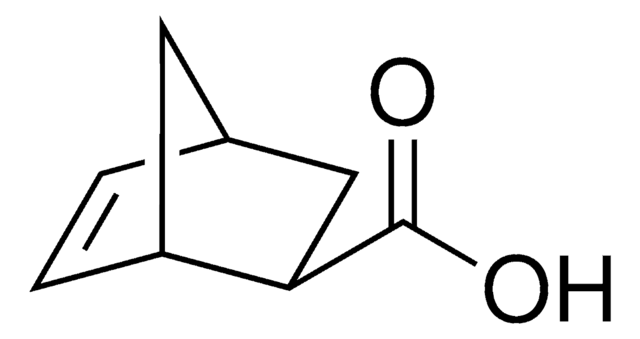
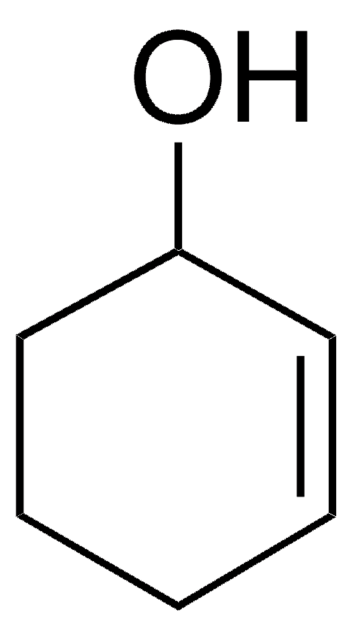
3-Cyclohexene-1-carboxylic acid
97%
Synonym(s):
1,2,3,6-Tetrahydrobenzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H9CO2H
CAS Number:
Molecular Weight:
126.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.48 (lit.)
bp
130-133 °C/4 mmHg (lit.)
mp
17 °C (lit.)
density
1.081 g/mL at 25 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)C1CCC=CC1
InChI
1S/C7H10O2/c8-7(9)6-4-2-1-3-5-6/h1-2,6H,3-5H2,(H,8,9)
InChI key
VUSWCWPCANWBFG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The metabolism of cyclohexanecarboxylic acid and 3-cyclohexenecarboxylic acid by Pseudomonas putida.
E R Blakley et al.
Canadian journal of microbiology, 28(12), 1324-1329 (1982-12-01)
A strain of Pseudomonas putida grew rapidly on cyclohexanecarboxylic acid as a sole source of carbon. A CoA-mediated beta-oxidation pathway was induced for the metabolism of the compound. The organism could not utilize 3-cyclohexenecarboxylic acid as a sole source of
Karine Barral et al.
Journal of medicinal chemistry, 48(2), 450-456 (2005-01-22)
Starting from commercially available (rac)-3-cyclohexene-1-carboxylic acid, a series of purine and pyrimidine cis-substituted cyclohexenyl and cyclohexanyl nucleosides were synthesized through a key Mitsunobu reaction. Antiviral evaluations were performed on HIV, coxsackie B3, and herpes viruses (HSV-1, HSV-2, VZV, HCMV). Three
Bromination of 3-cyclohexene-1-carboxylic acid, epoxydation of methyl 3-cyclohexene-1-carboxylate and opening of methyl cis-and trans-3, 4-epoxycyclohexane-1-carboxylate: Stereochemical results.
Bellucci G, et al.
Tetrahedron, 28(13), 3393-3399 (1972)
Yi Yang et al.
Environmental science & technology, 48(4), 2344-2351 (2014-02-01)
The effect of halides on organic contaminant destruction efficiency was compared for UV/H2O2 and UV/S2O8(2-) AOP treatments of saline waters; benzoic acid, 3-cyclohexene-1-carboxylic acid, and cyclohexanecarboxylic acid were used as models for aromatic, alkene, and alkane constituents of naphthenic acids
Total synthesis of (.+-.)-methyl shikimate and (.+-.)-3-phosphoshikimic acid.
Bartlett PA and McQuaid LA.
Journal of the American Chemical Society, 106(25), 7854-7860 (1984)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service