419885
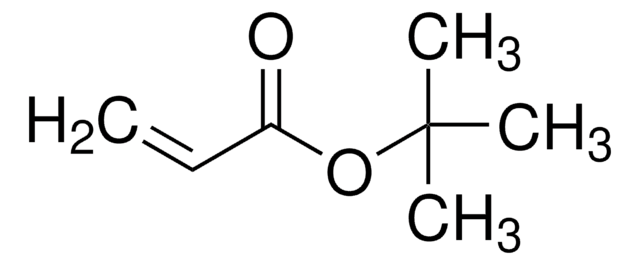
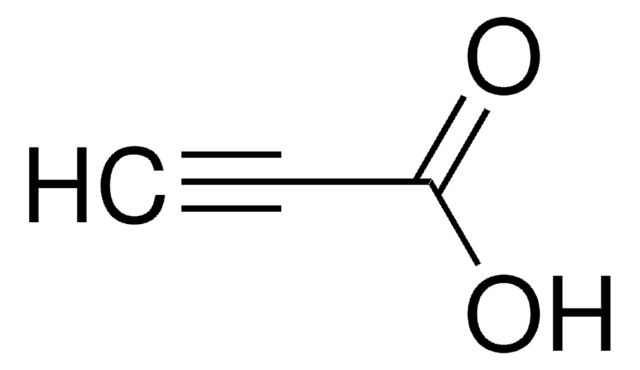
tert-Butyl propiolate
98%
Synonym(s):
tert-Butyl acetylenecarboxylate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
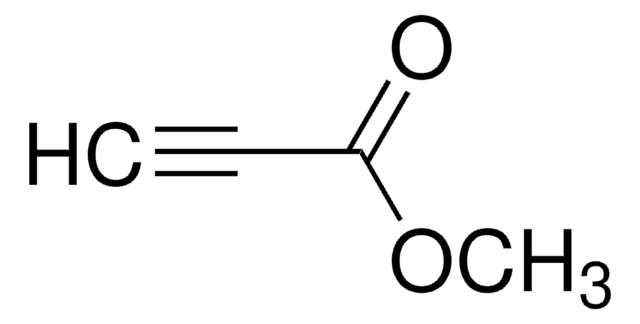
HC≡CCOOC(CH3)3
CAS Number:
Molecular Weight:
126.15
Beilstein:
1747175
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.418 (lit.)
bp
52-53 °C/27 mmHg (lit.)
mp
18-20 °C (lit.)
density
0.919 g/mL at 25 °C (lit.)
functional group
ester
SMILES string
CC(C)(C)OC(=O)C#C
InChI
1S/C7H10O2/c1-5-6(8)9-7(2,3)4/h1H,2-4H3
InChI key
XGTPDIIFEPTULX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
tert-Butyl propiolate is an ester. It reacts with methimazole to afford tert-butyl (E)-3-(1-methyl-1H-imidazol-2-ylthio) acrylate. Crystallographic data for the lithium enolate of tert-butyl propiolate has been described..
Application
tert-Butyl propiolate may be used in the preparation of heterocycles, alkaloids, and unsaturated amino acids.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
82.4 °F - closed cup
Flash Point(C)
28 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron, 48, 669-669 (1992)
Structures of three lithium ester enolates by X-ray diffraction: derivation of reaction path for cleavage into ketene and alcoholate.
Seebach D, et al.
Journal of the American Chemical Society, 170(19), 5403-5409 (1985)
Tetrahedron Letters, 30, 3625-3625 (1989)
Christopher M Hattan et al.
Synthetic communications, 43(1), 1-8 (2013-01-01)
The syntheses of 3-(1-methyl-1
Journal of the American Chemical Society, 112, 7682-7682 (1990)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 419885-1G | 4061832092751 |
| 419885-250MG |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service