419869
Ethynyl p-tolyl sulfone
98%
Synonym(s):
p-Toluenesulfonylacetylene, Tosylacetylene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
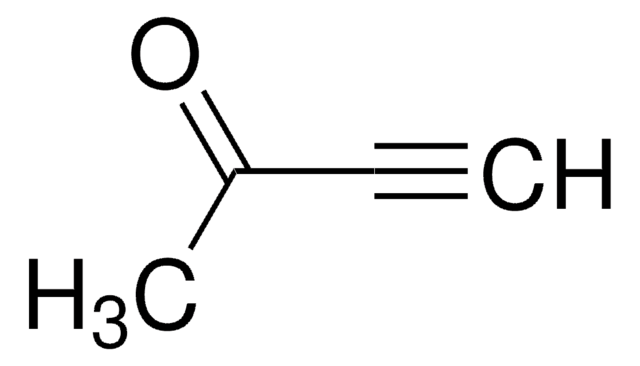
CH3C6H4SO2C≡CH
CAS Number:
Molecular Weight:
180.22
Beilstein:
2556169
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
73-74 °C (lit.)
solubility
organic solvents: soluble(lit.)
SMILES string
Cc1ccc(cc1)S(=O)(=O)C#C
InChI
1S/C9H8O2S/c1-3-12(10,11)9-6-4-8(2)5-7-9/h1,4-7H,2H3
InChI key
FTHKWIMQNXVEHW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Ethynyl p-tolyl sulfone (Tosylacetylene) may be used in the synthesis of 2-(4-methylphenylsulfonyl)ethenyl (tosvinyl, Tsv) protected derivatives. It may be used as starting reagent for the synthesis of optically active indan-2-ols.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Takeshi Hanazawa et al.
The Journal of organic chemistry, 68(12), 4980-4983 (2003-06-07)
An efficient and practical synthesis of optically active indan-2-ols 1 has been developed starting from readily accessible optically active 4-siloxy-1,6-alkadiynes 2 and ethynyl p-tolyl sulfone, where the metalative Reppe reaction mediated by an economical divalent titanium reagent, Ti(O-i-Pr)(4)/2 i-PrMgCl, is
A Simplified Method for the Preparation of Ethynyl P-Tolyl Sulfone and Ethynyl Phenyl Sulfone.
Chen Z and Trudell ML.
Synthetic Communications, 24(21), 3149-3155 (1994)
David Tejedor et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(66), 15046-15049 (2019-09-26)
A universal, practical and scalable organocatalytic hydrocyanation manifold to provide β-substituted acrylonitriles bearing an electron-withdrawing functionality has been implemented. The catalytic manifold operates under the reactivity generation principle "a good nucleophile generates a strong base", and it uses 1,4-diazabicyclo[2.2.2]octane (DABCO)
Elena Petit et al.
The Journal of organic chemistry, 79(18), 8826-8834 (2014-08-28)
The use of the 2-(4-methylphenylsulfonyl)ethenyl (tosvinyl, Tsv) group for the protection of the NH group of a series of imides, azinones (including AZT), inosines, and cyclic sulfonamides has been examined. The Tsv-protected derivatives are obtained in excellent yields by conjugate
Hao Chen et al.
Bioorganic & medicinal chemistry letters, 17(7), 1979-1983 (2007-02-16)
A library of potential antifungal triazole-modified beta-methoxyacrylate analogues was designed and synthesized via a Cu(I)-catalyzed 1,3-dipolar alkyne-azide coupling reaction or 'click chemistry'. Subsequent biological screening revealed that some compounds displayed low to moderate antifungal activity toward pathogenic fungi and low
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service