412295
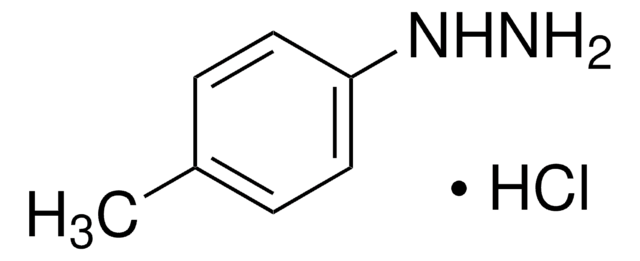
4-(Trifluoromethyl)phenylhydrazine
96%
Synonym(s):
1-(4-Trifluoromethylphenyl)hydrazine, 4-Trifluoromethylphenylhydrazine, [4-(Trifluoromethyl)phenyl]hydrazine, p-Trifluoromethylphenylhydrazine
About This Item
Recommended Products
Quality Level
Assay
96%
bp
118-122 °C/17 mmHg (lit.)
mp
63-65 °C (lit.)
functional group
fluoro
hydrazine
storage temp.
2-8°C
SMILES string
NNc1ccc(cc1)C(F)(F)F
InChI
1S/C7H7F3N2/c8-7(9,10)5-1-3-6(12-11)4-2-5/h1-4,12H,11H2
InChI key
DBNLGTYGKCMLLR-UHFFFAOYSA-N
Related Categories
General description
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 412295-5G | 4061832099958 |
| 412295-1G |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service