367141
Selenophene
97%
Synonym(s):
Selenacyclopentadiene, Selenofuran
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H4Se
CAS Number:
Molecular Weight:
131.03
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.58 (lit.)
bp
110-111 °C (lit.)
mp
−30 °C (lit.)
density
1.423 g/mL at 25 °C (lit.)
SMILES string
c1cc[se]c1
InChI
1S/C4H4Se/c1-2-4-5-3-1/h1-4H
InChI key
MABNMNVCOAICNO-UHFFFAOYSA-N
General description
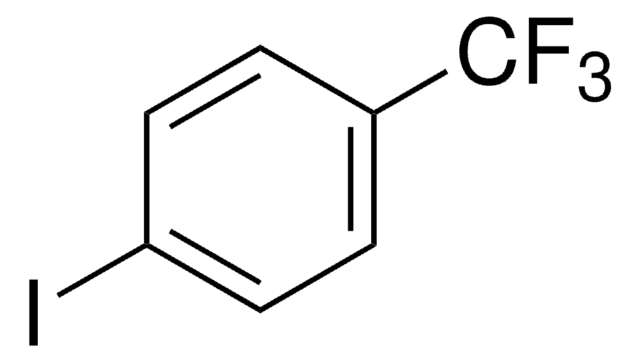
Selenophene is a heterocyclic building block. Synthesis of selenophene-based heteroacenes has been reported. Synthesis of selenophene-thiophene block copolymers has been reported. The electron transmission spectra of selenophene has been recorded in the 0-6eV energy range.
Application
Selenophene may be used:
- as conjugated linker in the synthesis of organic dyes
- in the synthesis of selenophene-2-carbonitrile
- in the synthesis of selenophene diketopyrrolopyrrole polymers
- as building block for the electrically conducting polyalkyl selenophene
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Flam. Liq. 2 - STOT RE 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
26.6 °F - closed cup
Flash Point(C)
-3 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Low band gap selenophene-diketopyrrolopyrrole polymers exhibiting high and balanced ambipolar performance in bottom-gate transistors.
Shahid M, et al.
Chemical Science, 3(1), 181-185 (2012)
Journal of the Electrochemical Society, 137, 1827-1827 (1990)
Dye-sensitized solar cells based on organic sensitizers with different conjugated linkers: furan, bifuran, thiophene, bithiophene, selenophene, and biselenophene.
Li R, et al.
The Journal of Physical Chemistry C, 113(17), 7469-7479 (2009)
Electron transmission spectra of selenophene and tellurophene and Xa computations of electron affinities for chalcophenes.
Modelli A, et al.
Chemical Physics, 88(3), 181-185 (1984)
Jon Hollinger et al.
Journal of the American Chemical Society, 132(25), 8546-8547 (2010-06-08)
Selenophene-thiophene block copolymers were synthesized and studied. The properties of these novel block copolymers are distinct from those of statistical copolymers prepared from the same monomers with a similar composition. Specifically, the block copolymers exhibit broad and red-shifted absorbance features
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service