359963
Boron trifluoride dihydrate
96%
Synonym(s):
Boron fluoride dihydrate, Trifluoroborane dihydrate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
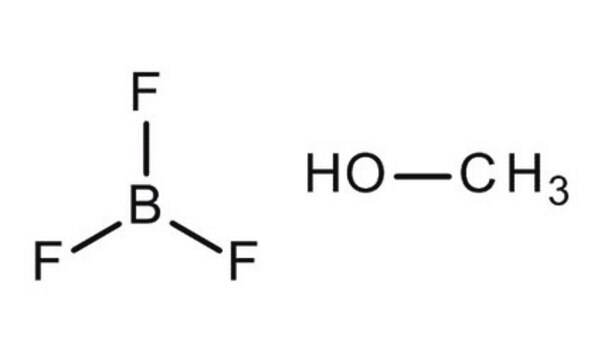
BF3 · 2H2O
CAS Number:
Molecular Weight:
103.84
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
reaction suitability
core: boron
reagent type: Lewis acid
reagent type: catalyst
refractive index
n20/D 1.316 (lit.)
density
1.636 g/mL at 25 °C (lit.)
SMILES string
[H]O[H].[H]O[H].FB(F)F
InChI
1S/BF3.2H2O/c2-1(3)4;;/h;2*1H2
InChI key
MJCYPBSRKLJZTB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Boron trifluoride dihydrate can be used as:
- An activator in Pechmann condensation to synthesize 4-methylcoumarin derivatives by the reaction between substituted phenols and methyl acetoacetate.
- An initiator for the polymerization of benzoxazine.
- A catalyst in the regioselective Fries rearrangement of phenolic esters to give hydroxyphenyl alkyl/aryl ketones.
- A catalyst to prepare 8-hydroxymethyltricyclene acetate by the reaction between camphene and formaldehyde in the presence of methylene chloride-acetic anhydride solvent.
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Terpene-formaldehyde reactions. II. d-Limonene
Blomquist AT and Himics RJ
The Journal of Organic Chemistry, 33(3), 1156-1159 (1968)
Cationic polymerization of benzoxazine monomers by boron trifluoride complex.
Cid JA, et al.
Polymers & Polymer Composites, 7(6), 409-420 (1999)
Pechmann reaction promoted by boron trifluoride dihydrate.
Stoyanov E and Mezger J.
Molecules (Basel), 10(7), 762-766 (2005)
Diego Méndez et al.
Biochemical pharmacology, 183, 114341-114341 (2020-11-17)
Platelets are the smallest blood cells, and their activation (platelet cohesion or aggregation) at sites of vascular injury is essential for thrombus formation. Since the use of antiplatelet therapy is an unsolved problem, there are now focused and innovative efforts
Yanfeng Liu et al.
Toxicology in vitro : an international journal published in association with BIBRA, 65, 104778-104778 (2020-01-22)
The need of in vitro alternative methods has been increasing in toxicology research as well as in cosmetic industry in China recently. Following the establishment of China EpiSkin™ skin corrosion and irritation testing methods, both as stand-alone in vitro tests
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service