All Photos(1)
About This Item
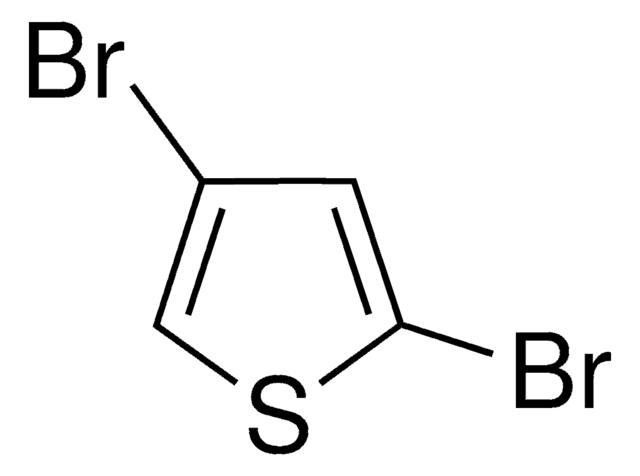
Empirical Formula (Hill Notation):
C4H2I2S
CAS Number:
Molecular Weight:
335.93
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
139-140 °C (lit.)
mp
37-41 °C (lit.)
functional group
iodo
storage temp.
2-8°C
SMILES string
Ic1ccc(I)s1
InChI
1S/C4H2I2S/c5-3-1-2-4(6)7-3/h1-2H
InChI key
PNYWRAHWEIOAGK-UHFFFAOYSA-N
General description
The multilayer desorption behavior of 2,5-diidothiophene was studied.
Application
2,5-Diiodothiophene was used in the preparation of oligothiophene films. It was used in maskless fabrication of periodic patterns of a conjugated polymer. It was also used in fabrication of oligothiophene and polythiophene micropatterns.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Photochemical production of oligothiophene and polythiophene micropatterns from 2, 5-diiodothiophene on Au in UHV.
Liu G, et al.
Surface Science, 592(1), L305-L309 (2005)
Guangming Liu et al.
The journal of physical chemistry. B, 110(41), 20197-20201 (2006-10-13)
The multilayer desorption behavior of 2,5-diidothiophene and the dendritic aggregation of photochemical reaction products during the desorption of 2,5-diiodothiophene multilayers have been studied. Like many other aromatic compounds, 2,5-diiodothiophene shows a multilayer desorption behavior different from the typical zeroth-order kinetics
Sudarshan Natarajan et al.
The journal of physical chemistry. B, 110(15), 8047-8051 (2006-04-14)
This paper describes the details of surface reactions producing >100-nm-thick conjugated polymer films. When 2,5-diiodothiophene films deposited on copper are irradiated with UV at room temperature in Ar environments, oligothiophene films are synthesized. The average conjugation length of the produced
Sudarshan Natarajan et al.
Langmuir : the ACS journal of surfaces and colloids, 21(15), 7052-7056 (2005-07-13)
Maskless fabrication of periodic patterns of a conjugated polymer is achieved by regioselective condensation of 2,5-diiodothiophene on chemically patterned substrate surfaces followed by in situ photochemical conversion of the condensed molecules into oligothiophenes and polythiophenes. This approach utilizes preferential aggregation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service