320129
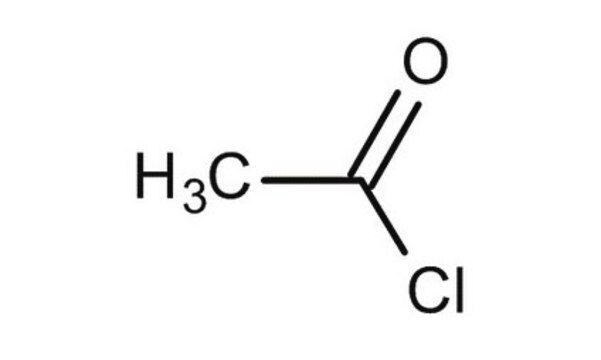
Acetyl chloride
reagent grade, 98%
Synonym(s):
Acetic acid chloride, Acetic chloride, Ethanoyl chloride
About This Item
Recommended Products
grade
reagent grade
Quality Level
vapor density
2.7 (vs air)
vapor pressure
11.69 psi ( 20 °C)
32.33 psi ( 55 °C)
Assay
98%
form
liquid
autoignition temp.
1353 °F
expl. lim.
19 %
refractive index
n20/D 1.389 (lit.)
bp
52 °C (lit.)
mp
−112 °C (lit.)
density
1.104 g/mL at 25 °C (lit.)
SMILES string
CC(Cl)=O
InChI
1S/C2H3ClO/c1-2(3)4/h1H3
InChI key
WETWJCDKMRHUPV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Synthesis, Characterization, and Evaluation of Thiazolidine Derivatives of Cysteine for Suppressing Eumelanin Production.: The study discusses the synthesis and evaluation of thiazolidine derivatives of cysteine, where acetyl chloride is used as a reagent, underscoring its importance in pharmaceutical intermediate development. (Amino et al., 2016).
- New URJC-1 Material with Remarkable Stability and Acid-Base Catalytic Properties.: This research introduces the new URJC-1 material, noting its stability and catalytic properties, with acetyl chloride being pivotal in the synthesis process, illustrating its role in material science and catalysis. (Leo et al., 2016).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
41.0 °F - closed cup
Flash Point(C)
5 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The Friedel–Crafts acylation is the reaction of an arene with acyl chlorides or anhydrides using a strong Lewis acid catalyst. This reaction proceeds via electrophilic aromatic substitution to form monoacylated products.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service