30740
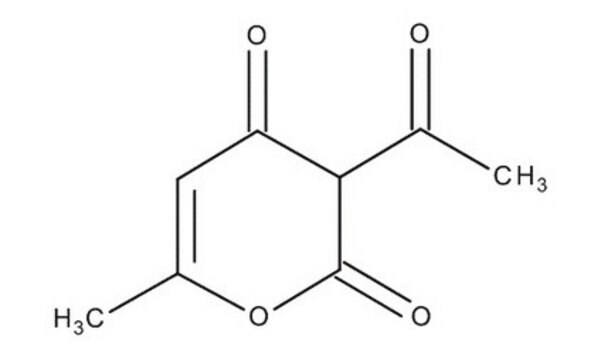
Dehydroacetic acid
≥98.0% (T)
Synonym(s):
2-Acetyl-5-hydroxy-3-oxo-4-hexenoic acid δ-lactone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H8O4
CAS Number:
Molecular Weight:
168.15
Beilstein:
6129
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (T)
form
powder
bp
270 °C (lit.)
mp
111-113 °C (lit.)
functional group
ester
ketone
SMILES string
CC(=O)C1C(=O)OC(C)=CC1=O
InChI
1S/C8H8O4/c1-4-3-6(10)7(5(2)9)8(11)12-4/h3,7H,1-2H3
InChI key
PGRHXDWITVMQBC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Dehydroacetic acid is one of the food additives found in red wine and was determined by ultra-fast liquid chromatography-tandem quadrupole mass spectrometry (UFLC-MS/MS).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
314.6 °F
Flash Point(C)
157 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xiao-Hong Chen et al.
Journal of chromatography. A, 1263, 34-42 (2012-10-10)
A novel and effective dispersive solid-phase extraction (dSPE) procedure with rapid magnetic separation using ethylenediamine-functionalized magnetic polymer as an adsorbent was developed. The new procedure had excellent clean-up ability for the selective removal of the matrix in red wine. An
Ségolène De Vaugelade et al.
Rapid communications in mass spectrometry : RCM, 32(11), 862-870 (2018-03-10)
The present work is devoted to the structural elucidation of by-products issued from the direct ultraviolet-visible (UV-vis) irradiation of dehydroacetic acid (DHA) in solution and in cosmetic emulsion. Analyses were carried out using gas chromatography coupled with ion trap mass
Yansheng Wang et al.
Journal of food science, 75(6), M383-M388 (2010-08-21)
The peptide mixture from housefly pupae has broad spectrum antimicrobial activity but has not previously been reported as a food preservative. In this study, the preservation effects of a housefly pupae peptide mixture, nisin, and sodium dehydroacetate (DHA-S) on the
L Zema et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 75(2), 291-296 (2010-03-23)
A delivery device intended for the prolonged release of antimicrobial agents, able to enhance the stability profile of liquid/semi-solid cosmetic/pharmaceutical products for topical application, was proposed in the present study. With the aid of a simulation program based on compartment
Takashi Ohtsuki et al.
Food chemistry, 141(2), 1322-1327 (2013-06-26)
An absolute quantification method for the determination of dehydroacetic acid in processed foods using quantitative (1)H NMR was developed and validated. The level of dehydroacetic acid was determined using the proton signals of dehydroacetic acid referenced to 1,4-bis (trimethylsilyl) benzene-d4
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service