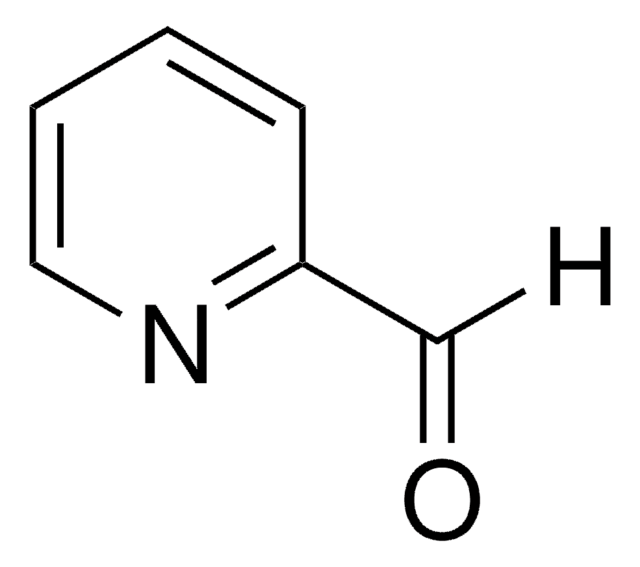
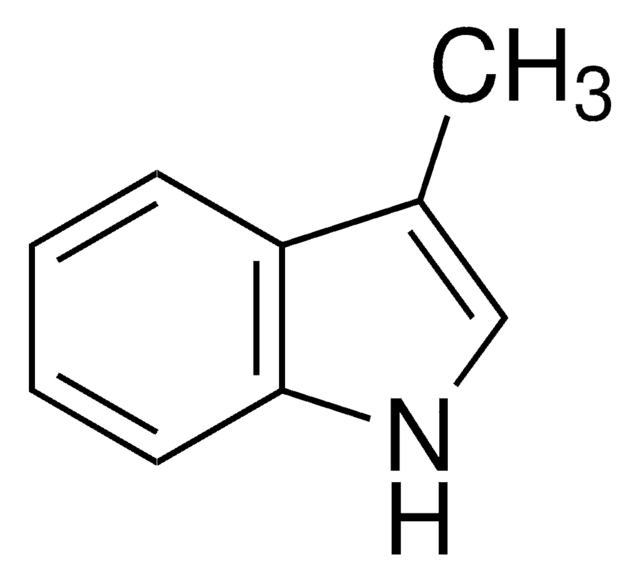
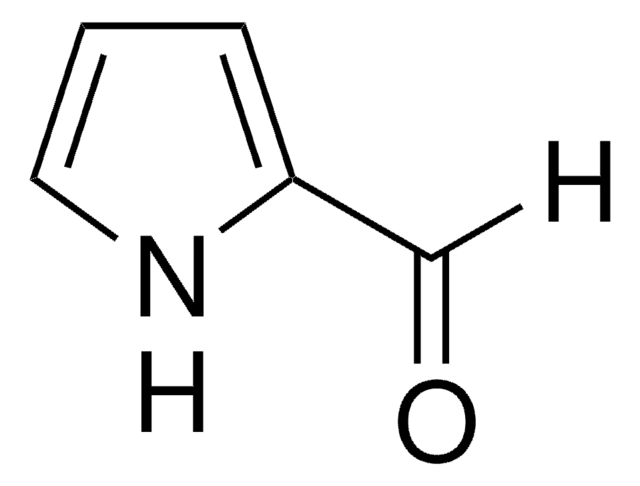
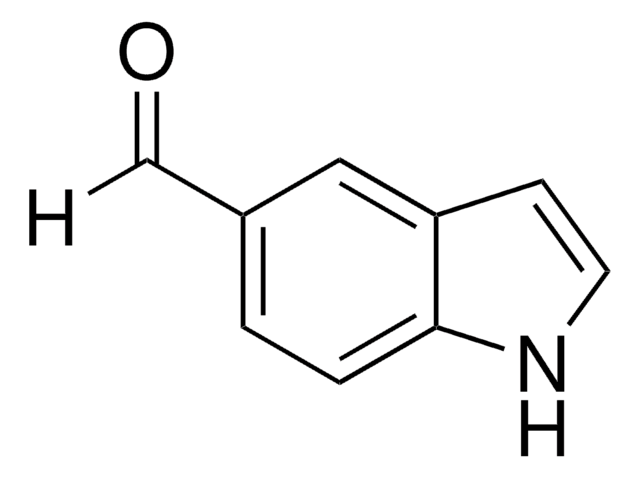
262439
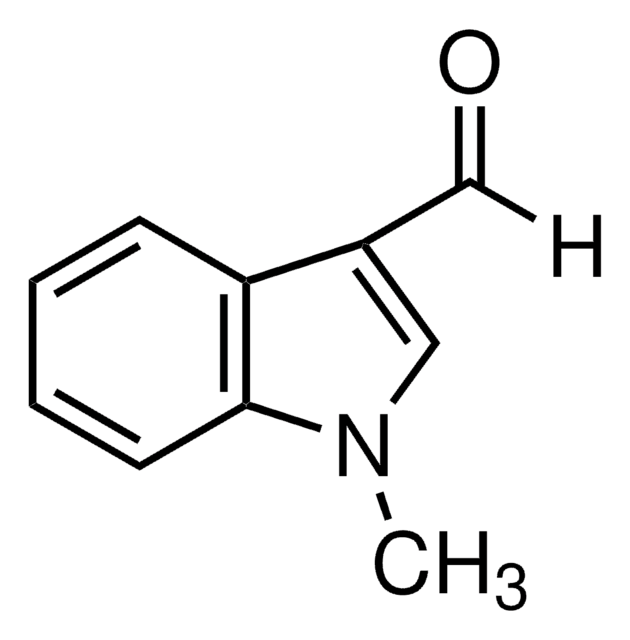
2-Methylindole-3-carboxaldehyde
97%
Synonym(s):
3-Formyl-2-methylindole, NSC 11895
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H9NO
CAS Number:
Molecular Weight:
159.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
200-201 °C (lit.)
SMILES string
Cc1[nH]c2ccccc2c1C=O
InChI
1S/C10H9NO/c1-7-9(6-12)8-4-2-3-5-10(8)11-7/h2-6,11H,1H3
InChI key
CYZIVXOEJNAIBS-UHFFFAOYSA-N
General description
Oxidative activation of 2-methylindole-3-carboxaldehyde via N-heterocyclic carbene organocatalysis generates heterocyclic ortho-quinodimethane as a key intermediate.
Application
2-Methylindole-3-carboxaldehyde has been used in the preparation of 1-phenylsulfonyl-2-methylindole-3-carboxaldehyde.
Reactant for preparation of:
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Fluorescent sensors (BODIPY)
- Antimicrobial agents against methicillin-resistant Staphylococcus aureus
- G protein-coupled receptor CRTh2 antagonists
- Inhibitors of PI3 kinase-α
- Antitubercular agents
- Anti-inflammatory agents
- Mycobacterium tuberculosis protein tyrosine phosphatase B
- Glucocorticoid receptor ligands
- Agents stimulating neurite outgrowth
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xingkuan Chen et al.
Angewandte Chemie (International ed. in English), 52(42), 11134-11137 (2013-09-17)
Aryl aldehyde activation: Oxidative activation of 2-methylindole-3-carboxaldehyde (I) through N-heterocyclic carbene (NHC) organocatalysis generates heterocyclic ortho-quinodimethane (II) as a key intermediate. This intermediate then undergoes formal [4+2] cycloaddition with trifluoromethyl ketones or isatins to form polycyclic lactones containing a quaternary
G Chakkaravarthi et al.
Acta crystallographica. Section E, Structure reports online, 64(Pt 2), o542-o542 (2008-01-01)
In the title compound, C(16)H(15)NO(3)S, the plane of the phenyl ring forms a dihedral angle of 80.37 (8)° with the indole ring system. The crystal packing is stabilized by weak O-H⋯O hydrogen bonds which link the mol-ecules into infinite chains along
Ming-Zhi Zhang et al.
European journal of medicinal chemistry, 92, 776-783 (2015-01-31)
Streptochlorin, first isolated as a new antibiotic in 1988 from the lipophilic extracts of the mycelium of a Streptomyces sp, is an indole natural products with a variety of biological activities. Based on the methods developed for the synthesis of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service