All Photos(1)
About This Item
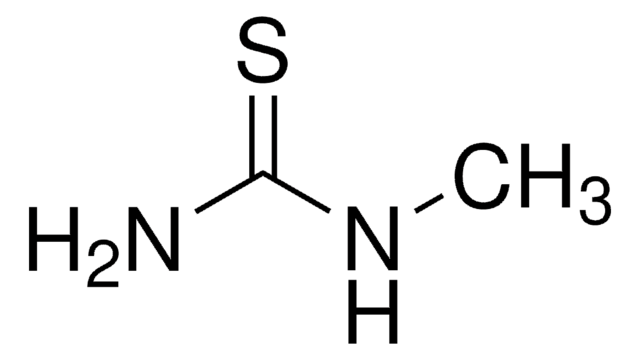
Linear Formula:
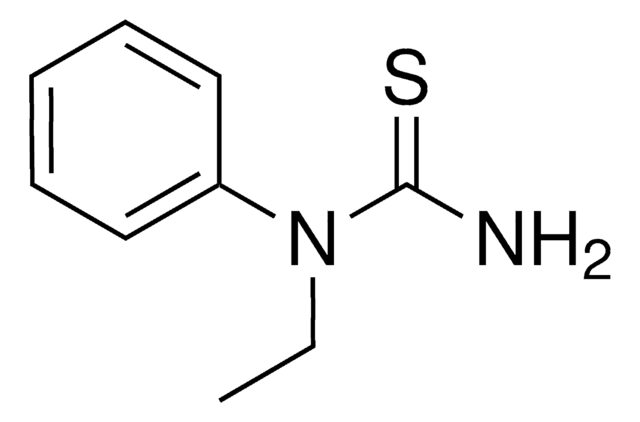
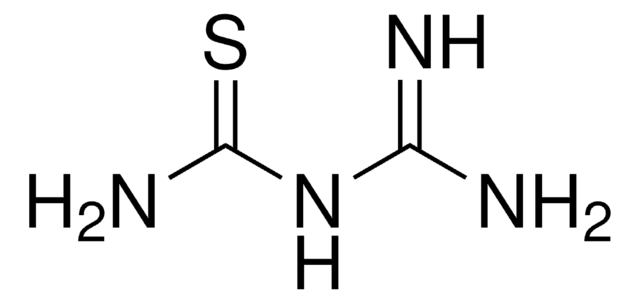
C2H5NHCSNH2
CAS Number:
Molecular Weight:
104.17
Beilstein:
1699551
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
108-110 °C (lit.)
solubility
methanol: soluble 25 mg/mL, clear, colorless
functional group
amine
thiourea
SMILES string
CCNC(N)=S
InChI
1S/C3H8N2S/c1-2-5-3(4)6/h2H2,1H3,(H3,4,5,6)
InChI key
GMEHFXXZSWDEDB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N-Ethylthiourea forms adduct with silver halides and their single crystal X-ray structural and spectroscopic characterizations are described.
Application
N-Ethylthiourea was used in the preparation of Re (III) complexes, which are precursors for the new rhenium complexes, potentially useful in nuclear medicine. It was also used in two-step protocol for the first chemoselective cleavage of 2-hydroxy acid amides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Erwin P Schreiner et al.
The Journal of organic chemistry, 67(24), 8299-8304 (2002-11-26)
A two-step protocol for the first chemoselective cleavage of 2-hydroxy acid amides has been developed. Mesylation of the model substrate 2-(hydroxypropionylamino)-4-methylpentanoic acid methyl ester (11) followed by treatment with N-ethylthiourea (13) allows cleavage of 2-hydroxy acid amides under smooth conditions.
Dinorah Gambino et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 58(14), 3085-3092 (2003-01-04)
Complexes of the type [Re(III)L6]X3, with L = thiourea, N-methylthiourea, N-ethylthiourea or N,N'-dimethytlthiourea and X = Cl- or PF6-, were prepared as suitable precursors for the synthesis of new rhenium complexes potentially useful in nuclear medicine. The infrared (IR) spectra
M N Preobrazhenskaya et al.
The Journal of antibiotics, 44(2), 192-199 (1991-02-01)
A series of phenylthiourea and ethylthiourea derivatives of daunorubicin and its congeners was prepared by reaction of the 3'-amino group of the antibiotic with phenylisothiocyanate or ethylisothiocyanate. S-Methylation yielded S-methylisothiouromium salts which when reacted with amines resulted in an intramolecular
Graham A Bowmaker et al.
Dalton transactions (Cambridge, England : 2003), 39(18), 4391-4404 (2010-04-28)
Syntheses, single crystal X-ray structural and spectroscopic characterizations are described for a variety of adducts of silver halides with thiourea ('tu'), N-ethylthiourea ('ettu' = EtNH.CS.NH(2)) and N,N'-diethylthiourea ('detu' = EtNH.CS.EtNH). This study greatly extends our knowledge of the complex chemistry
Xutong Sun et al.
American journal of respiratory cell and molecular biology, 50(6), 1084-1095 (2014-01-08)
Recent studies have indicated that, during the development of pulmonary hypertension (PH), there is a switch from oxidative phosphorylation to glycolysis in the pulmonary endothelium. However, the mechanisms underlying this phenomenon have not been elucidated. Endothelin (ET)-1, an endothelial-derived vasoconstrictor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service