219878
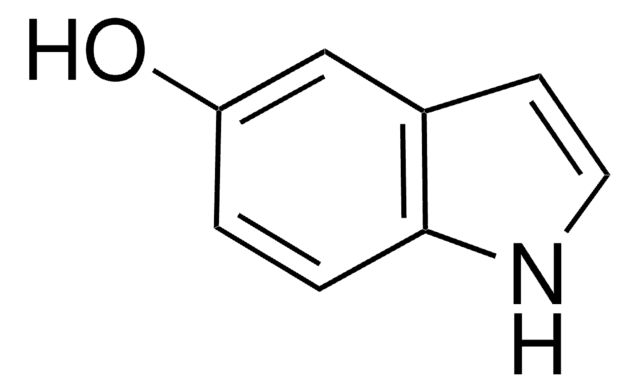
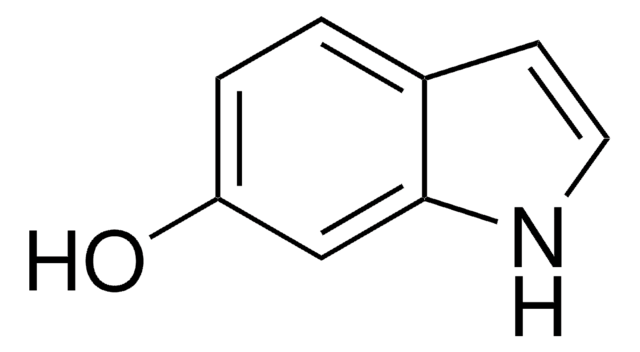
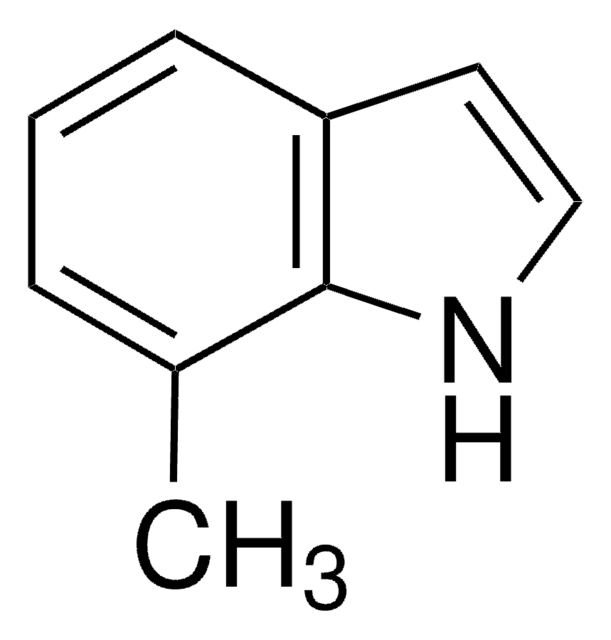
4-Hydroxyindole
99%
Synonym(s):
4-Indolol, 4-Hydroxyindole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H7NO
CAS Number:
Molecular Weight:
133.15
Beilstein:
114905
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
97-99 °C (lit.)
SMILES string
Oc1cccc2[nH]ccc12
InChI
1S/C8H7NO/c10-8-3-1-2-7-6(8)4-5-9-7/h1-5,9-10H
InChI key
NLMQHXUGJIAKTH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Glucouridination of 4-hydroxyindole by human uridine 5′-diphospho-glucuronosyltransferase has been repotrted.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stefan W Toennes et al.
Journal of pharmaceutical and biomedical analysis, 140, 215-222 (2017-04-04)
Each year, synthetic cannabinoids are occurring in high numbers in the illicit drug market, but data on their pharmacology and toxicology are scarcely available. Therefore, a pilot study was performed to assess adverse effects of JWH-018, which is one of
Gavin Carr et al.
Journal of medicinal chemistry, 51(9), 2634-2637 (2008-04-09)
Synthetic analogues of the sponge natural product exiguamine A (3) have been prepared and evaluated for their ability to inhibit indoleamine 2,3-dioxygenase in vitro.
Shalenie P den Braver-Sewradj et al.
Current drug metabolism, 19(4), 370-381 (2018-01-11)
Inter-individual variability in hepatic drug metabolizing enzyme (DME) activity is a major contributor to heterogeneity in drug clearance and safety. Accurate data on expression levels and activities of DMEs is an important prerequisite for in vitro-in vivo extrapolation and in
Alexandra M Adams et al.
Metabolic engineering, 56, 111-119 (2019-09-25)
Psilocybin, the prodrug of the psychoactive molecule psilocin, has demonstrated promising results in clinical trials for the treatment of addiction, depression, and post-traumatic stress disorder. The development of a psilocybin production platform in a highly engineerable microbe could lead to
Marek Bednarski et al.
Archiv der Pharmazie, 349(3), 211-223 (2016-02-09)
β-Adrenergic receptor antagonists are important therapeutics for the treatment of cardiovascular disorders. In the group of β-blockers, much attention is being paid to the third-generation drugs that possess important ancillary properties besides inhibiting β-adrenoceptors. Vasodilating activity of these drugs is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service