212806
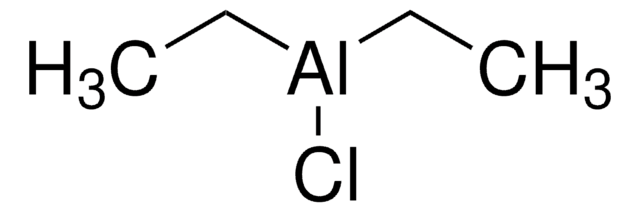
Diethylaluminum chloride solution
1.0 M in hexanes
Synonym(s):
Chlorodiethylaluminum, Dichlorotetraethyldialuminium, Diethylaluminum monochloride
About This Item
Recommended Products
form
liquid
Quality Level
concentration
1.0 M in hexanes
density
0.711 g/mL at 25 °C
SMILES string
CC[Al](Cl)CC
InChI
1S/2C2H5.Al.ClH/c2*1-2;;/h2*1H2,2H3;;1H/q;;+1;/p-1
InChI key
YNLAOSYQHBDIKW-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- As a Lewis acid for the synthesis of Lewis acid-base bifunctional asymmetric catalysts for cyanosilylation of aldehydes and in Strecker reaction.
- For the synthesis of precursors for lanthanide/aluminum catalysts for diene polymerization.
- As a co-initiator in the cationic polymerization of isobutylene.
Packaging
Legal Information
Signal Word
Danger
Hazard Statements
Hazard Classifications
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Repr. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 1
Target Organs
Central nervous system
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 3
Flash Point(F)
-7.6 °F
Flash Point(C)
-22 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service