All Photos(2)
About This Item
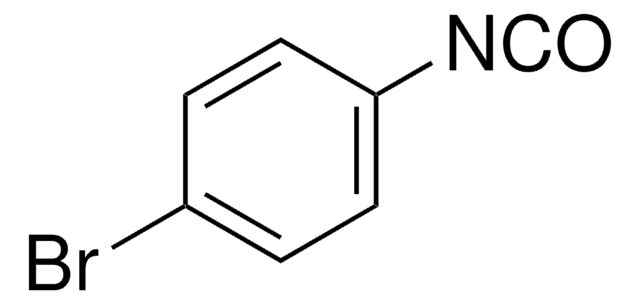
Linear Formula:
OCNC6H4CO2C2H5
CAS Number:
Molecular Weight:
191.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
bp
118-119 °C/0.8 mmHg (lit.)
mp
27-29 °C (lit.)
functional group
ester
storage temp.
2-8°C
SMILES string
CCOC(=O)c1ccc(cc1)N=C=O
InChI
1S/C10H9NO3/c1-2-14-10(13)8-3-5-9(6-4-8)11-7-12/h3-6H,2H2,1H3
InChI key
CFEPCPHKICBCJV-UHFFFAOYSA-N
Application
Ethyl 4-isocyanatobenzoate was used in the preparation of cellulose carbamate and ester derivatives. It was also used in the preparation of ethyl 4-(3-(4-oxo-6-tridecyl-1,4-dihydropyrimidin-2-yl)ureido)benzoate and ethyl 4-(2-oxocyclohexanecarboxamido)benzoate.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
>230.0 °F - closed cup
Flash Point(C)
> 110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Electronic substituent effects on hydrogen-bonding motifs modulate supramolecular polymerisation.
Pellizzaro ML, et al.
Royal Society of Chemistry Advances, 3(9), 3103-3108 (2013)
Cellulose derivatives synthesized via isocyanate and activated ester pathways in homogeneous solutions of lithium chloride/N, N-dimethylacetamide.
Williamson SL and McCormick CL.
J. Macromol. Sci., Pure Appl. Chem., 35(12), 1915-1927 (1998)
Chun-Ho Park et al.
Bioorganic & medicinal chemistry letters, 20(7), 2250-2253 (2010-03-02)
Highly potent poly(ADP-ribose)polymerase-1 (PARP-1) inhibitors, including 9-hydroxy-1,2-dihydro-4H-thiopyrano[3,4-c]quinolin-5(6H)-one derivatives with a non-aromatic A-ring, were synthesized. Among the derivatives, 12a showed low nanomolar enzyme and cellular activity (IC(50) = 42 nM, ED(50) = 220 nM) with good water solubility. Further, 12a exhibited
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service