All Photos(3)
About This Item
Empirical Formula (Hill Notation):
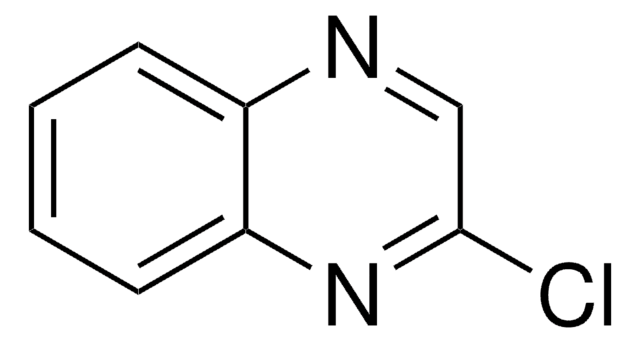
C8H4Cl2N2
CAS Number:
Molecular Weight:
199.04
Beilstein:
126076
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
solid
mp
152-154 °C (lit.)
functional group
chloro
SMILES string
Clc1nc2ccccc2nc1Cl
InChI
1S/C8H4Cl2N2/c9-7-8(10)12-6-4-2-1-3-5(6)11-7/h1-4H
InChI key
SPSSDDOTEZKOOV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,3-Dichloroquinoxaline reacts with 6-aminothiouracil in ethanol/TEA to form 6-amino-2-(3-chloroquinoxalin-2-ylthio)pyrimidin-4(3H)-one. It reacts with cholest-5-en-3-one semicarbazone/thiosemicarbazone to form steriodal cholest-5-en-3-oxazolo and thiazoloquinoxaline.
Application
2,3-Dichloroquinoxaline was used in the synthesis of mono and 2,3-disubstituted quinoxalines. It was used in solid phase synthesis of HPLC chiral stationary phase containing the N,N′-dialkyl-2,3-diaminoquinoxaline group as a linking structure.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Performance of a new HPLC chiral stationary phase derived from N-(3, 5-dinitrobenzoyl)-D-alpha-phenylglycine with a quinoxaline branching unit.
Forjan DM, et al.
Acta Chromatographica , 17, 97-97 (2006)
K Shiva Kumar et al.
Bioorganic & medicinal chemistry, 20(5), 1711-1722 (2012-02-10)
A direct and single-step method has been developed for the synthesis of mono and 2,3-disubstituted quinoxalines by using a AlCl(3) induced (hetero)arylation of 2,3-dichloroquinoxaline. Both symmetrical and unsymmetrical 2,3-disubstituted quinoxalines can be prepared conveniently by using this method under appropriate
Shadia A Galal et al.
European journal of medicinal chemistry, 46(1), 327-340 (2010-12-15)
The reaction of o-phenylene diamine and ethyl oxamate is reinvestigated and led to 3-aminoquinoxalin-2(1H)-one rather than benzimidazole-2-carboxamide as was previously reported. The structure of the obtained quinoxaline has been confirmed by X-ray. The anti-tumor activity of synthesized quinoxalines 1-21 has
A A Abu-Hashem et al.
European journal of medicinal chemistry, 45(5), 1976-1981 (2010-02-13)
Treatment of 6-aminothiouracil (1) with 2,3-dichloroquinoxaline (2) in ethanol/TEA afforded 6-amino-2-(3-chloroquinoxalin-2-ylthio)pyrimidin-4(3H)-one (3), which was refluxed in DMF to give 2-aminopyrimido[2',1':2,3]thiazolo[4,5-b]quinoxaline-4-one (4). Compound 4 was utilized as a key intermediate for the synthesis of a new pyrimido[2',1':2,3]thiazolo[4,5-b]quinoxaline derivatives 5-14via the reaction
Salman Ahmad Khan et al.
European journal of medicinal chemistry, 43(10), 2257-2261 (2008-04-29)
Some heterocyclic systems namely cholest-5-en-7-thiazolo[4,5-b]quinoxaline-2-yl-hydrazone] were synthesized by the reaction of cholest-5-en-7-one-thiosemicarbazone with 2,3-dichloroquinoxaline at 80 degrees C in high yield. The thiosemicarbazone derivatives were obtained by the condensation of the thiosemicarbazide with steroidal ketones. All the compounds have been
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service