143685
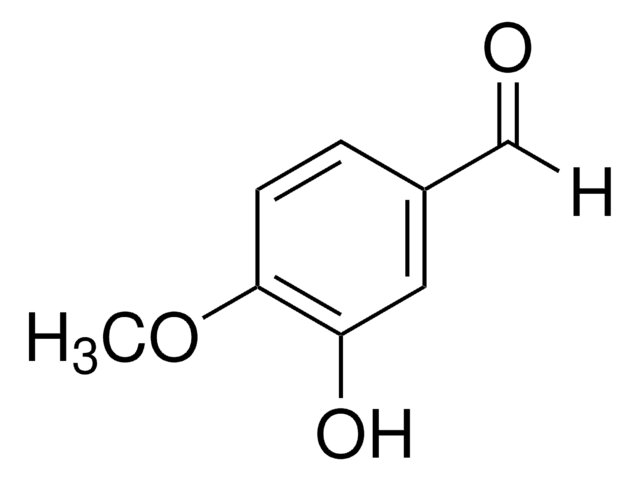
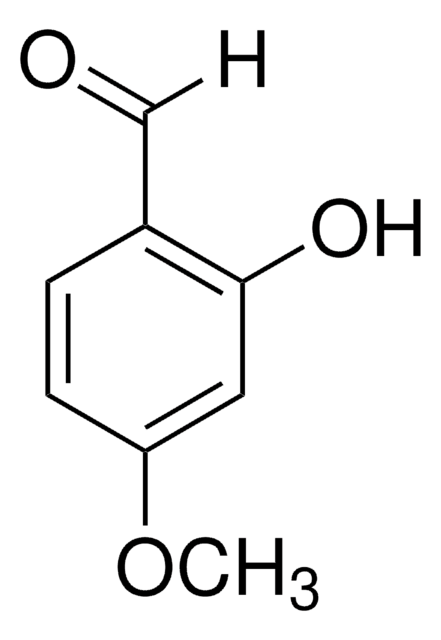
3-Hydroxy-4-methoxybenzaldehyde
99%
Synonym(s):
3-Hydroxyanisaldehyde, Isovanillin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H3(OCH3)CHO
CAS Number:
Molecular Weight:
152.15
Beilstein:
1073021
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
bp
179 °C/15 mmHg (lit.)
mp
113-115 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1ccc(OC)c(O)c1
InChI
1S/C8H8O3/c1-11-8-3-2-6(5-9)4-7(8)10/h2-5,10H,1H3
InChI key
JVTZFYYHCGSXJV-UHFFFAOYSA-N
General description
3-Hydroxy-4-methoxybenzaldehyde on condensation with furan-2-carboxylic acid hydrazide and thiophene-2-carboxylic acid hydrazide yields Schiff-bases. It undergoes condensation reaction with1-azabicyclo[2.2.2]octan-3-one to give (Z)-2-(3-hydroxy-4-methoxybenzylidene)-1-azabicyclo[2.2.2]octan-3-one.
Application
3-Hydroxy-4-methoxybenzaldehyde was used as starting reagent during the two-step stereoselective synthesis of the anticancer drug (Z)-combretastatin A-4 and glycitein synthesis.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
>212.0 °F
Flash Point(C)
> 100 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Caroline Lang'at-Thoruwa et al.
Journal of natural products, 66(1), 149-151 (2003-01-25)
4-Methoxyresorcinol (3) was synthesized as the precursor for glycitein (6) synthesis by the oxidation of 3-hydroxy-4-methoxybenzaldehyde (1) to the aryl formate with H2O2 and a catalytic amount of SeO2. Glycitein (6) was synthesized by cyclization of 2,4,4'-trihydroxy-5-methoxydeoxybenzoin (5) with N,N-dimethylformamide
K Gaukroger et al.
The Journal of organic chemistry, 66(24), 8135-8138 (2001-11-28)
A high-yielding, two-step stereoselective synthesis of the anticancer drug (Z)-combretastatin A-4 (1) has been devised. The method uses the Perkin condensation of 3,4,5-trimethoxyphenylacetic acid and 3-hydroxy-4-methoxybenzaldehyde followed by decarboxylation of the cinnamic acid intermediate using copper and quinoline. The iodine-catalyzed
Vijayakumar N Sonar et al.
Acta crystallographica. Section C, Crystal structure communications, 59(Pt 11), o647-o649 (2003-11-08)
Crystals of the title compound, C(15)H(17)NO(3), were obtained from a condensation reaction of 3-hydroxy-4-methoxybenzaldehyde with 1-azabicyclo[2.2.2]octan-3-one and subsequent crystallization of the product from methanol. The title compound, containing a double bond that connects the azabicyclic ring system to the 3-hydroxy-4-methoxybenzylidene
Riyadh M Ahmed et al.
TheScientificWorldJournal, 2013, 754868-754868 (2013-09-13)
New monomeric cobalt and cadmium complexes with Schiff-bases, namely, N'-[(E)-(3-hydroxy-4-methoxyphenyl)methylidene]furan-2-carbohydrazide (L¹) and N'-[(E)-(3-hydroxy-4-methoxyphenyl)methylidene]thiophene-2-carbohydrazide (L²) are reported. Schiff-base ligands L¹ and L² were derived from condensation of 3-hydroxy-4-methoxybenzaldehyde (iso-vanillin) with furan-2-carboxylic acid hydrazide and thiophene-2-carboxylic acid hydrazide, respectively. Complexes of the
Michael D Markey et al.
Organic letters, 9(17), 3255-3257 (2007-07-31)
The first total synthesis of santiagonamine (1) is achieved in 12 steps from isovanillin. A palladium-catalyzed Ullmann cross-coupling reaction and a photocyclization are the key steps in the synthesis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service