136948
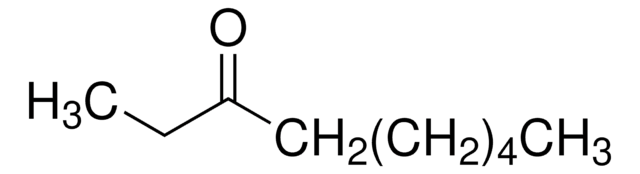
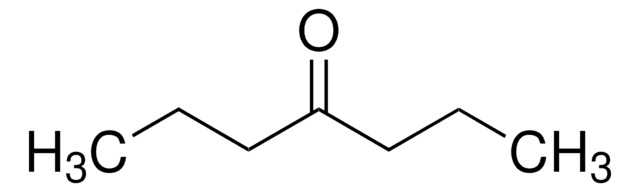
5-Nonanone
98%
Synonym(s):
Dibutyl ketone, Valerone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)3CO(CH2)3CH3
CAS Number:
Molecular Weight:
142.24
Beilstein:
1743583
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.419 (lit.)
bp
186-187 °C (lit.)
mp
−50 °C (lit.)
density
0.826 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
CCCCC(=O)CCCC
InChI
1S/C9H18O/c1-3-5-7-9(10)8-6-4-2/h3-8H2,1-2H3
InChI key
WSGCRAOTEDLMFQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
5-Nonanone can be used as a reactant to prepare:
- 5-nonanketoxime by reacting with hydroxylamine in water.
- 4-Nitro-2,6-dipropylphenol by treating with a solution of formyl nitroenamine in the presence of a base.
Biochem/physiol Actions
5-Nonanone induces clinical neuropathy in rats.
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
141.8 °F - closed cup
Flash Point(C)
61 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Commercial-grade methyl heptyl ketone (5-methyl-2-octanone) neurotoxicity: contribution of 5-nonanone.
J L O'Donoghue et al.
Toxicology and applied pharmacology, 62(2), 307-316 (1982-02-01)
Possible role of metabolism in 5-nonanone neurotoxicity.
G D DiVincenzo et al.
Neurotoxicology, 3(1), 55-63 (1982-07-01)
Controlling Selectivity in the Consecutive Reaction Network of Aldoxime Hydrogenation to Primary Amines.
Gebauer-Henke E, et al.
Catalysis Science & Technology, 2, 2539-2548 (2012)
Development of a general Pd (II)-catalyzed intermolecular hydroalkoxylation reaction of vinylphenols by using a sacrificial alcohol as the hydride source
Zhang Y and Sigman MS
Organic Letters, 8(24), 5557-5560 (2006)
Techno-economic analysis of 5-nonanone production from levulinic acid.
Patel AD, et al.
Chemical Engineering Journal, 160(1), 311-321 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service