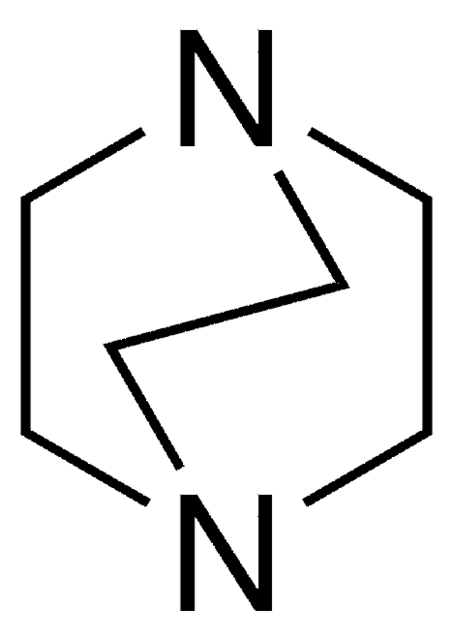
135895
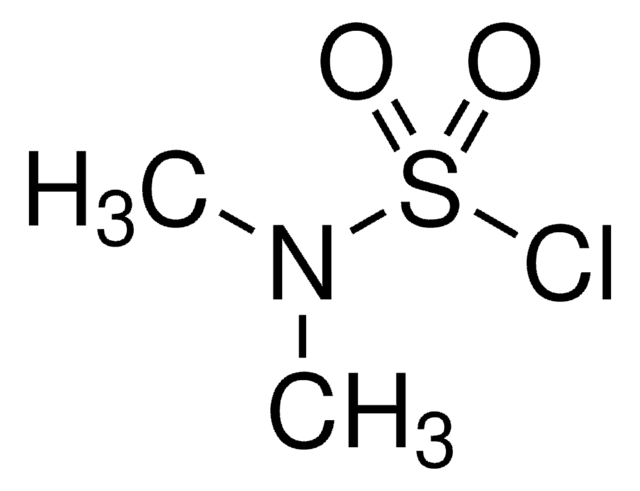
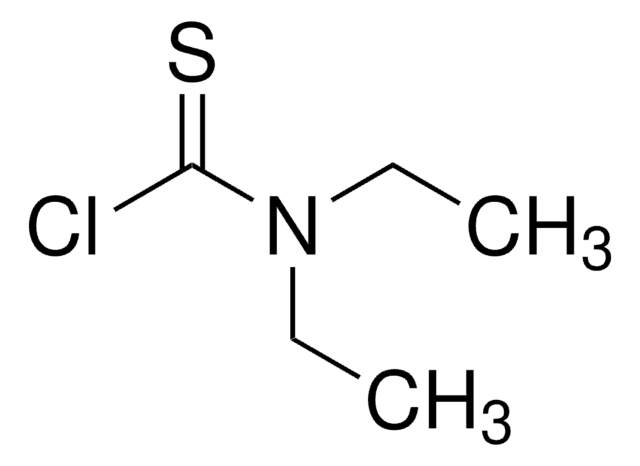
Dimethylthiocarbamoyl chloride
97%
Synonym(s):
1-Chloro-N,N-dimethylthioformamide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)2NCSCl
CAS Number:
Molecular Weight:
123.60
Beilstein:
635823
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
bp
90-95 °C/0.5 mmHg (lit.)
mp
39-43 °C (lit.)
solubility
chloroform: soluble 100 mg/mL, clear to very slightly hazy, very faintly yellow
functional group
amine
chloro
storage temp.
2-8°C
SMILES string
CN(C)C(Cl)=S
InChI
1S/C3H6ClNS/c1-5(2)3(4)6/h1-2H3
InChI key
PHWISQNXPLXQRU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Dimethylthiocarbamoyl chloride was used in chemoselective deoxygenation of pyridine-N-oxides. It was used in the synthesis of (±)-thia-calanolide A. It was used as starting reagent in the synthesis of dimethylthiocarbamates.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
208.4 °F - closed cup
Flash Point(C)
98 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Journal of Heterocyclic Chemistry, 44, 487-487 (2007)
D K Barma et al.
Organic letters, 5(25), 4755-4757 (2003-12-05)
Dimethylthiocarbamates (DMTCs), prepared from the corresponding alcohols using commercial dimethylthiocarbamoyl chloride, are spectrally simple, achiral, and nonpolar. DMTCs are moderately to highly stable to a wide range of reagents and conditions including metal hydrides, hydroboration, ylides, NaOH, HCl, organolithiums, Grignards
A synthesis of (?)-thia-calanolide A, its resolution and in vitro biological evaluation.
Chopade AU, et al. et al.
Arabian Journal of Chemistry (2012)
Harley D Betts et al.
Molecules (Basel, Switzerland), 25(19) (2020-10-07)
Silver(I)-based coordination polymers or metal-organic frameworks (MOFs) display useful antibacterial properties, whereby distinct materials with different bonding can afford control over the release of silver(I) ions. Such silver(I) materials are comprised of discrete secondary building units (SBUs), and typically formed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![1,4-Diazabicyclo[2.2.2]octane for synthesis](/deepweb/assets/sigmaaldrich/product/images/488/587/f5a877b3-e573-4686-931f-648015f4d284/640/f5a877b3-e573-4686-931f-648015f4d284.jpg)