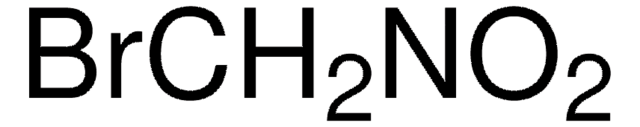125954
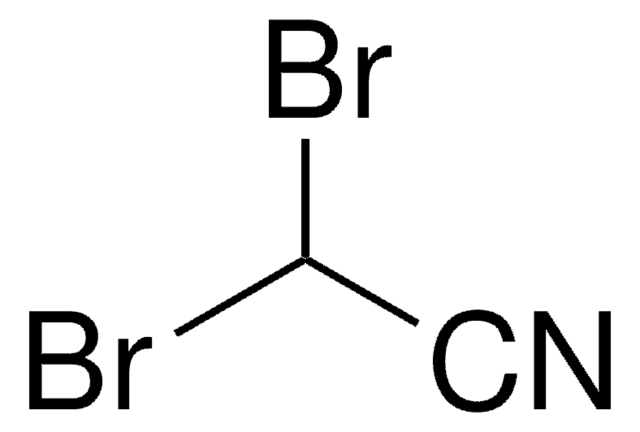
Dichloroacetonitrile
98%
Synonym(s):
2,2-Dichloroacetonitrile, Dichloromethyl cyanide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Cl2CHCN
CAS Number:
Molecular Weight:
109.94
Beilstein:
1739029
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.44 (lit.)
bp
110-112 °C (lit.)
density
1.369 g/mL at 25 °C (lit.)
SMILES string
ClC(Cl)C#N
InChI
1S/C2HCl2N/c3-2(4)1-5/h2H
InChI key
STZZWJCGRKXEFF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Dichloroacetonitrile can be used as a reactant to prepare:
- Chiral α, α-dichloro-β-aminonitriles via Pd-catalyzed enantioselective Mannich-type reaction with imines.
- α, α-dialkyl-substituted nitriles by an alkylation reaction with trialkylboranes in the presence of phenoxide base as a base.
- Halogenated pyridines via copper-catalyzed reaction with methacrolein.
- α,α-dichloro-β-hydroxy nitriles by condensation reaction with aldehydes and ketones in the presence of an alkoxide base.
- Selenium heterocycle derivatives via Diels–Alder cyclization with selenoaldehydes.
- Dichloroacetonitrile can also be used to develop an efficient method for the extraction and determination of common volatile halogenated disinfection by-products using the static headspace technique coupled with gas chromatography-mass spectrometry.
Biochem/physiol Actions
Dichloroacetonitrile is direct-acting mutagen and induces DNA strand breaks in cultured human lymphoblastic cells. It induces apoptosis or necrosis in murine macrophage cell line via reactive oxygen intermediates-mediated oxidative mechanisms of cellular damage.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
96.8 °F - closed cup
Flash Point(C)
36 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Isabel Montesinos et al.
Journal of chromatography. A, 1310, 113-120 (2013-09-03)
A simple and efficient method has been developed for the extraction and determination of sixteen common volatile halogenated disinfection by-products (DBPs) using the static headspace (HS) technique coupled with gas chromatography-mass spectrometry (GC-MS). The DBPs determined included trihalomethanes (THMs), halonitromethanes
M K Smith et al.
Fundamental and applied toxicology : official journal of the Society of Toxicology, 12(4), 765-772 (1989-05-01)
Dichloroacetonitrile (DCAN), a by-product of drinking water disinfection formed by reaction of chlorine with background organic materials, was evaluated for its developmental effects in pregnant Long-Evans rats. Animals were dosed by oral intubation on Gestation Days 6-18 (plug = 0)
Dichloroacetonitrile
GL Bundy
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Speciation of common volatile halogenated disinfection by-products in tap water under different oxidising agents
Montesinos I and Gallego M
Journal of Chromatography A, 1310, 113-120 (2013)
M R Roby et al.
Environmental health perspectives, 69, 215-220 (1986-11-01)
The excretion and tissue distribution of [1-14C]dichloroacetonitrile and [2-14C]dichloroacetonitrile were studied in male Fischer 344 rats and male B6C3F1 mice. Three dose levels of dichloroacetonitrile (DCAN) (0.2, 2, or 15 mg/kg) were administered to rats and two dose levels of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
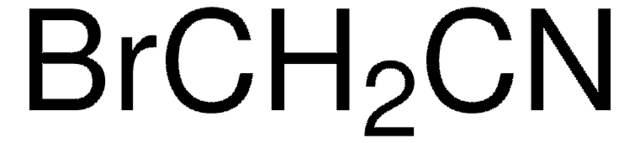



![Bicyclo[2.2.1]hept-2-ene 99%](/deepweb/assets/sigmaaldrich/product/structures/270/492/95fd4909-6108-4858-8c94-609b54387149/640/95fd4909-6108-4858-8c94-609b54387149.png)