123188
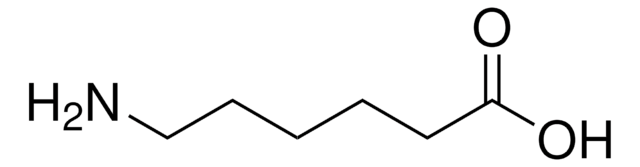
5-Aminovaleric acid
97%, for peptide synthesis
Synonym(s):
5-AVA, 5-Aminopentanoic acid, Homopiperidinic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
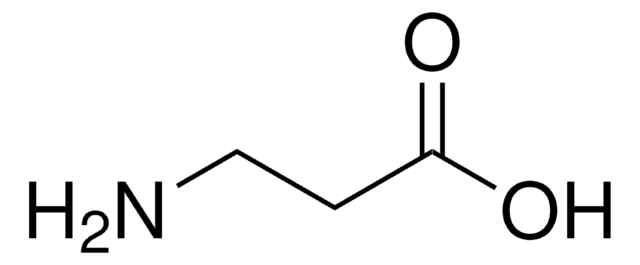
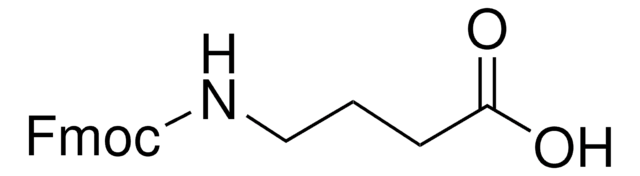
NH2(CH2)4CO2H
CAS Number:
Molecular Weight:
117.15
Beilstein:
906833
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Product Name
5-Aminovaleric acid, 97%
Quality Level
Assay
97%
form
solid
reaction suitability
reaction type: solution phase peptide synthesis
mp
158-161 °C (lit.)
application(s)
peptide synthesis
SMILES string
NCCCCC(O)=O
InChI
1S/C5H11NO2/c6-4-2-1-3-5(7)8/h1-4,6H2,(H,7,8)
InChI key
JJMDCOVWQOJGCB-UHFFFAOYSA-N
Gene Information
human ... SLC15A1(6564)
Looking for similar products? Visit Product Comparison Guide
Application
5-Aminovaleric acid (5-AVA) is used:
- In the preparation of (5-AVA)x(MA)1-xPbI3, a perovskite for fabricating printable mesoscopic perovskite solar cell.
- As a spacer in the synthesis of rhenium and technetium-99m labeled insulin.
- To synthesize dipeptides that self-assemble to form nanotubes in the solid state as well as in solution over a wide range of pH.
- As a starting material in the total synthesis of an alkaloid, lycoposerramine Z.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A hole-conductor?free, fully printable mesoscopic perovskite solar cell with high stability.
Mei A, et al.
Science, 345(6194), 295-298 (2014)
Dipeptide Nanotubes, with N-Terminally Located ω-Amino Acid Residues, That are Stable Proteolytically, Thermally, and Over a Wide Range of pH.
Guha S, et al.
Chemistry of Materials, 20(6), 2282-2290 (2008)
cis-Decahydroquinolines via asymmetric organocatalysis: Application to the total synthesis of lycoposerramine Z.
Bradshaw B, et al.
Organic Letters, 15(2), 326-329 (2012)
Synthesis and characterization of rhenium and technetium-99m labeled insulin.
Sundararajan C, et al.
Journal of Medicinal Chemistry, 53(6), 2612-2621 (2010)
Sylvain Poujol et al.
Clinical chemistry, 49(11), 1900-1908 (2003-10-28)
We developed gradient HPLC methods for quantification of the antimitotic drug irinotecan (CPT-11) and its four metabolites, SN-38, SN-38 G, 7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino]-carbonyloxycamptothecin (APC), and 7-ethyl-10-[4amino-1-piperidino]-carbonyloxycamptothecin (NPC), as the sum of the lactone and carboxylate forms, in human plasma and saliva.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 123188-25G | 4061838722140 |
| 123188-5G | 4061838722157 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service