121908
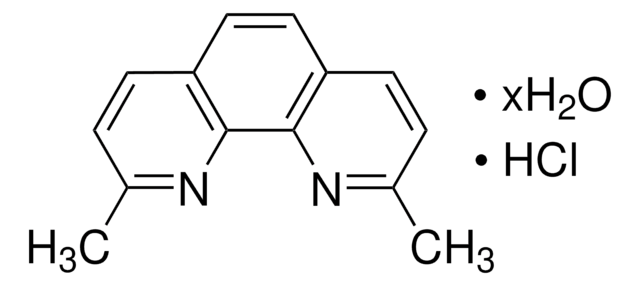
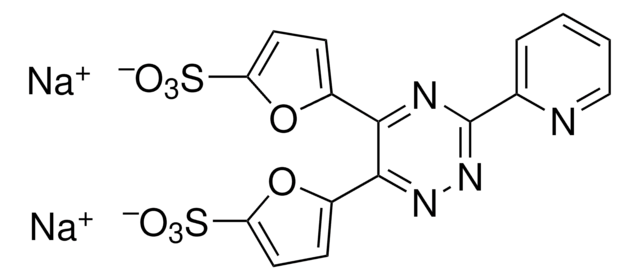
Neocuproine hydrate
99%
Synonym(s):
2,9-Dimethyl-1,10-phenanthroline hydrate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C14H12N2 · xH2O
CAS Number:
Molecular Weight:
208.26 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
solubility
methanol: soluble 50 mg/mL, clear, colorless to dark yellow
SMILES string
[H]O[H].Cc1ccc2ccc3ccc(C)nc3c2n1
InChI
1S/C14H12N2.H2O/c1-9-3-5-11-7-8-12-6-4-10(2)16-14(12)13(11)15-9;/h3-8H,1-2H3;1H2
InChI key
MFZBSWSCIWCRKS-UHFFFAOYSA-N
General description
Neocuproine hydrate is chelating agent and forms complex with Cu+ formed by reduction of Cu+2 by hydroxylamine hydrochloride and produces electrochemiluminescence. It is an efficient ligand for alcohol oxidation with palladium in 1:1 water/DMSO mixtures.
Application
Neocuproine hydrate was used in determination of copper ions in aqueous solution using electrochemiluminescence.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Determining copper ions in water using electrochemiluminescence.
High B, et al.
Analytica Chimica Acta, 449(1), 17-22 (2001)
Catalytic Conversions in Water. Part 23: Steric Effects and Increased Substrate Scope in the Palladium-Neocuproine Catalyzed Aerobic Oxidation of Alcohols in Aqueous Solvents#.
Brink GJ, et al.
Advanced Synthesis & Catalysis, 345(12), 1341-1352 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service