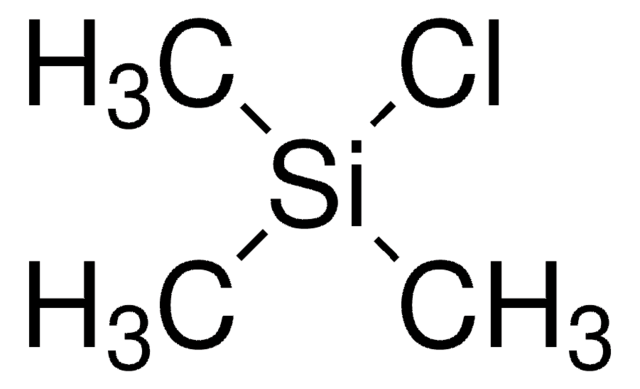113379
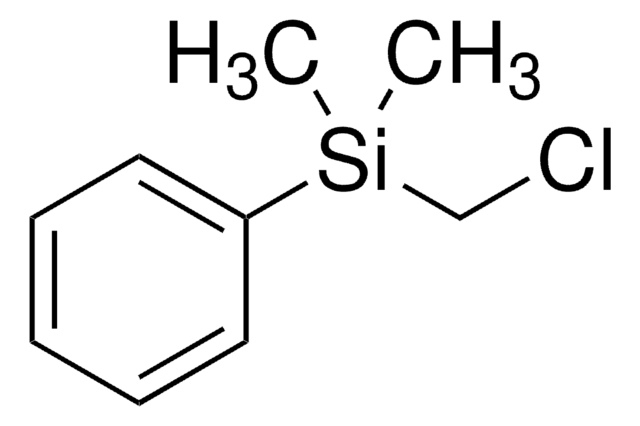
Chloro(dimethyl)phenylsilane
98%
Synonym(s):
DMPSCl, Dimethylphenylchlorosilane, Dimethylphenylsilylchloride, Phenyldimethylchlorosilane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5Si(CH3)2Cl
CAS Number:
Molecular Weight:
170.71
Beilstein:
606292
EC Number:
MDL number:
UNSPSC Code:
12352002
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.509 (lit.)
density
1.017 g/mL at 25 °C (lit.)
SMILES string
C[Si](C)(Cl)c1ccccc1
InChI
1S/C8H11ClSi/c1-10(2,9)8-6-4-3-5-7-8/h3-7H,1-2H3
InChI key
KWYZNESIGBQHJK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Used in a synthesis of enantioenriched allenylsilanes via an ortho-ester Claisen rearrangement of chiral, silylpropargylic alcohols.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
143.6 °F - closed cup
Flash Point(C)
62 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Carlton P Folster et al.
Dalton transactions (Cambridge, England : 2003), 49(45), 16125-16132 (2020-01-30)
The synthesis of a chloro-functionalized six-membered cyclosilazane (Si5N) is reported. Subsequent reductive polymerizations yielded low molecular weight polysilazanes. 1H and 29Si NMR characterization suggest the identity of the reducing metal influences the polysilazane structure. Optical characterization is consistent with extended
Ryan A Brawn et al.
Organic letters, 9(14), 2689-2692 (2007-06-15)
A convenient procedure for the synthesis of highly enantioenriched allenylsilanes by Johnson orthoester Claisen rearrangement of 1-silyl propargylic alcohols is described. Allenylsilanes are then used as carbon nucleophiles in three-component, Lewis acid mediated additions to in situ generated oxonium ions
M Okamoto
Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 112(6), 409-413 (1992-06-01)
The retention behavior and selectivity of 30 kinds of phenyl-modified porous glasses and silicas, prepared from solutions of phenyldimethylchlorosilane, diphenylmethylchlorosilane or triphenylchlorosilane in xylene, were studied by high-performance liquid chromatography using carbamazepine and diphenylhydantoin as model compounds. From the elemental
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service