112364
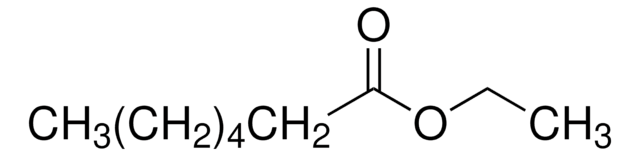
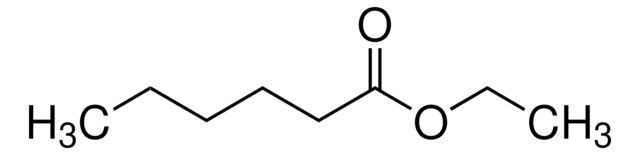
Ethyl heptanoate
ReagentPlus®, 99%
Synonym(s):
Ethyl enanthate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3(CH2)5COOC2H5
CAS Number:
Molecular Weight:
158.24
Beilstein:
1752311
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39022453
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
refractive index
n20/D 1.412 (lit.)
bp
188-189 °C (lit.)
mp
−66 °C (lit.)
density
0.87 g/mL at 25 °C (lit.)
functional group
ester
SMILES string
CCCCCCC(=O)OCC
InChI
1S/C9H18O2/c1-3-5-6-7-8-9(10)11-4-2/h3-8H2,1-2H3
InChI key
TVQGDYNRXLTQAP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Ethyl heptanoate, an aroma compound, was released from a series of sodium caseinate-stabilized, n-eicosane emulsions during the investigation of solid and liquid lipid droplet concentration.
Application
Ethyl heptanoate was used in a nickel nanoparticles-catalysed, transfer hydrogenation of olefins.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
165.2 °F - closed cup
Flash Point(C)
74 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Transfer hydrogenation of olefins catalysed by nickel nanoparticles.
Alonso F, et al.
Tetrahedron, 65(51), 10637-10643 (2009)
Supratim Ghosh et al.
Journal of agricultural and food chemistry, 54(5), 1829-1837 (2006-03-02)
Aroma compounds partition between the dispersed and the continuous phases in emulsions, and phase transitions in the lipid droplets profoundly affect the position of the equilibrium. In the present study, the release of ethyl butyrate, ethyl pentanoate, ethyl heptanoate, and
Monographs on fragrance raw materials.
D L Opdyke
Food and cosmetics toxicology, 19(2), 237-254 (1981-04-01)
Mohammad Rafienia et al.
Current drug delivery, 6(2), 184-191 (2009-05-20)
In situ forming biodegradable polymeric systems were prepared from Poly (DL-lactide-co-glycolide), RG504H (50:50, lactide:glycolide), RG756 (75:25) and mixture of them. They were dissolved in N-methyl-2-pyrrolidone (33% w/w) and mixed with betamethasone acetate (BTMA, 5 and 10% w/w) and ethyl heptanoate
J Enrique Cometto-Muñiz et al.
Behavioural brain research, 156(1), 115-123 (2004-10-12)
The investigation explored the olfactory detectability of two chemically and structurally similar esters, ethyl propanoate and ethyl heptanoate, presented singly and in mixtures. Initially, we measured concentration-detection (i.e., psychometric) functions for the odor of ethyl propanoate and ethyl heptanoate presented
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service