Using Activated Carbon as a Precious Metal Catalyst Carrier
Howard Wisniowski, Yong Zhang
Cabot Norit Activated Carbon, Cabot Corporation
Most chemical processes require a catalyst, making them vital to the pharmaceutical and fine chemical industry. Activated carbon is a material that has all the required characteristics to be used as a catalyst support. When compared to other carriers, like silica or alumina, activated carbons provide:
- Greater internal surface area to have higher reaction rate (as shown in Figure 1) and lower cost per cubic meter
- Stable inertness in harsh process conditions such as acidic and alkaline solutions
- Minimized interference with catalysis selectivity or activity due to the interest of carbon in most reactions
- Available in powder, granular, and extruded shapes
- Easy recovery of precious metals
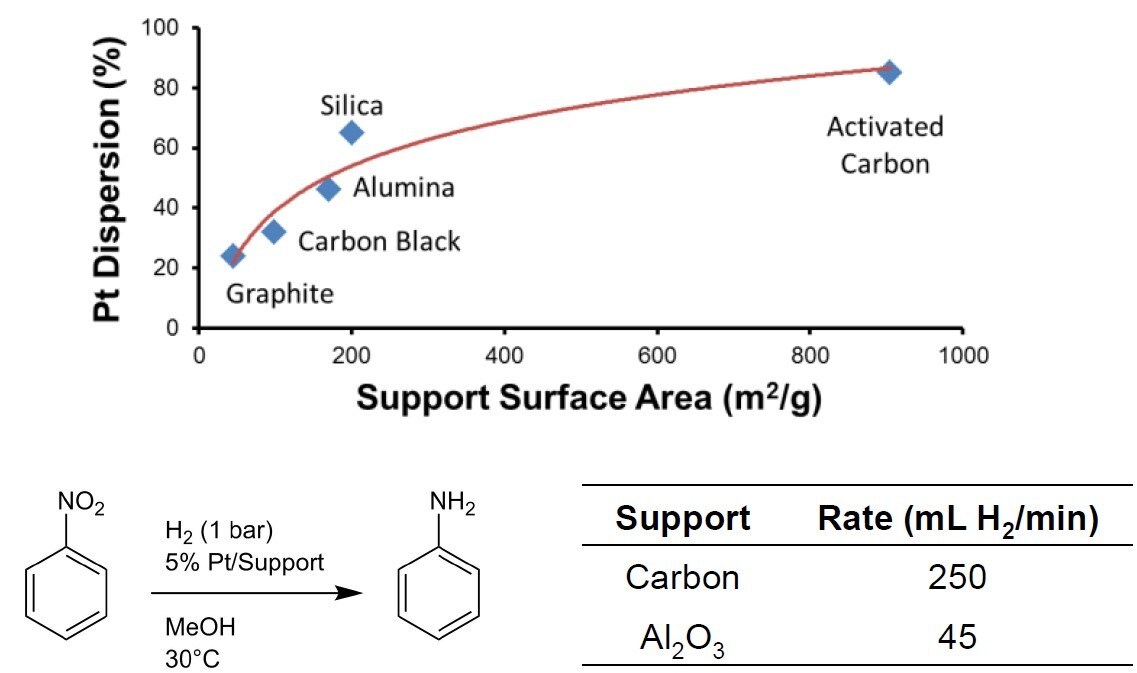
Figure 1. Comparison of different commercial precious metal catalyst carrier materials1
Cabot Norit activated carbon catalyst carriers offer consistency, high purity, low attrition, and unique surface chemistry. These products are available in various pore size distributions, resulting in excellent catalyst dispersion over the carbon surface.
What is Activated Carbon
Activated carbon is a highly porous, high surface-area adsorptive material with a largely amorphous structure. It is composed primarily of aromatic configurations of carbon atoms joined by random cross-linkages. Activated carbon differs from another form of carbon — graphite — in that activated carbon has sheets or groups of atoms that are stacked unevenly in a disorganized manner. The degree of order varies based on the starting raw material and thermal history. Graphitic platelets in steam-activated coal are somewhat ordered, while more amorphous aromatic structures are found in chemically activated wood.
Randomized bonding creates a highly porous structure with numerous cracks, crevices, and voids between the carbon layers. The molecular size, porosity, and the resulting enormous internal surface area of activated carbon make this material extremely effective for adsorbing a wide range of impurities from both liquids and gases. To provide some perspective for the internal surface area of activated carbon, the annual activated carbon production of Cabot Norit has a surface area nearly equal to the total land area on Earth (148 million km²). Figure 2 shows two micrographs of the internal structure of activated carbon based on steam-activated lignite.
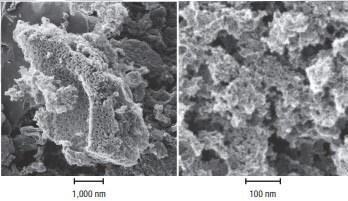
Figure 2.These images from a helium-ion microscope show the pore structure of lignite-based activated carbon
Activated carbon sorbents are tailored for specific applications mainly based on pore size and pore volume requirements. Porosity and other parameters are controlled by raw material selection, activation process conditions, and the post-processing steps. Depending on the application, activated carbon may be in the form of powder (PAC), granule (GAC) or extrudate (EAC). All three forms are available in a range of particle sizes (Figure 3).

Figure 3. Forms of activated carbon.


Activated Carbon as Catalyst Support
Many of today’s chemical processes require the use of a catalyst supported on a carrier. The greater internal surface area, high inertness and versatility make our activated carbons an ideal support in many applications including:
- Precious metal catalysts (e.g. Au, Pt, Pd, Ir, Ru, Rh, etc.)
- Base metal catalysts (e.g. Ni, Co, Cu, Zn, Fe)
Precious Metal Catalyst Support for Pharmaceutical and Fine Chemical Synthesis
Most pharmaceutical and fine chemical companies require catalysts for chemical synthesis. Cabot Norit activated carbon products play a key role as carriers for precious metals in these applications, offering strict product specifications, increasing the efficiency of the catalyst impregnation process and reducing valuable testing and process time.
Typical Chemical Reaction Applications
- Hydrogenation (e.g. Scheme 1)
- Hydrodehalogenation (e.g Scheme 2)
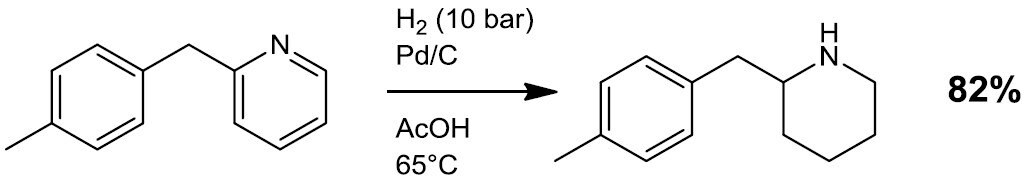
- Reductive alkylation/amination (e.g. Scheme 3)
- Carbon-oxygen/nitrogen cleavage
- Decarbonylation
- Disproportionation
- Dehydrogenation
- Dehydrohalogenation
- Debenzylation (e.g. Scheme 4)
- Oxidation (e.g. Scheme 5)
- Hydroxylamine synthesis
- Electro-catalysis
Scheme 1.2
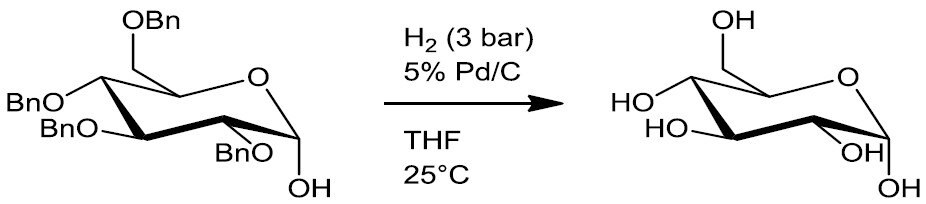
Scheme 4.5
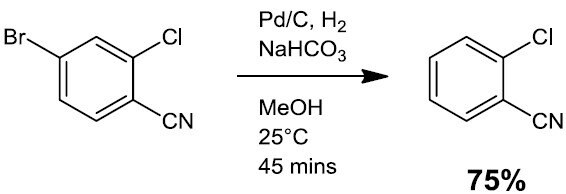
Scheme 2.3
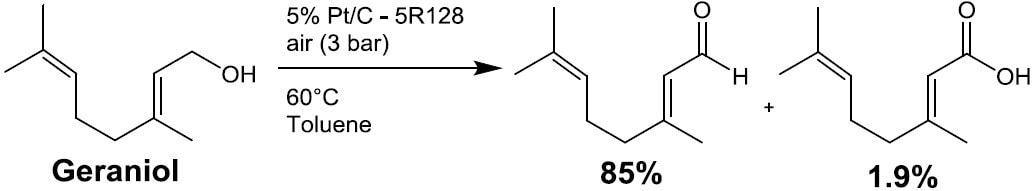
Scheme 5. 6
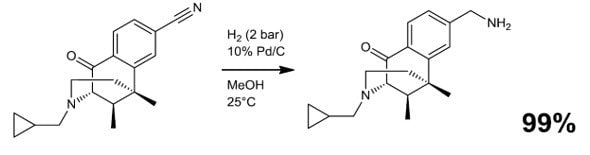
Scheme 3.4
Choosing the Right Activated Carbon
Due to high performance requirements in complex technical processes, only the highest quality activated carbons are selected for catalyst applications. Cabot Norit activated carbon products meet these requirements by providing the optimal purity, pore volume, form, hardness, and surface functionality necessary, making them the ideal choice for catalyst applications. Figure 4 summarizes properties of some of our activated carbon materials used for precious metal catalyst carrier applications.
Powdered Activated Carbon Performance Requirements
Kinetics and filterability
This is achieved by controlling particle size distribution. A broader particle size distribution gives better kinetics between reactants and the actual catalyst. A narrow particle size will give fast filtration but sacrifice suspension characteristics.
Optimal catalyst activities
Higher mesoporosity will give benefits in terms of available surface area for catalyst dispersion, faster transport of reactants in and out the pores, and eventual adsorption of unwanted byproducts.
Minimizing side reactions
High purity of the activated carbon is required to prevent side reaction or poisoning of the catalyst.
Extruded Activated Carbon Performance Requirements
Minimizing metal loss
A high crushing strength is required to prevent collapse of the carbon bed. High hardness results in a low abrasion to avoid catalyst into fines so that it minimizes the loss of your precious metals.
Longer life and higher yield
Effectively overcoming the poisoning of the actual catalyst will extend the catalyst’s life span.
Higher activity
This is achieved by the larger surface area of carbons with higher mesoporosity. In this situation the “egg shell” metal catalyst is mainly dispersed on the outside of the carbon particle.
References
Para continuar lendo, faça login ou crie uma conta.
Ainda não tem uma conta?