The Use of Perovskite Metal Complexes in Photovoltaic Cells
Avery Luedtke, Dr.
Sigma-Aldrich Materials Science
Introduction
Solar panels for homes and businesses have seen a rise in demand over the past few years as we move toward more environment-friendly and sustainable energy sources. In 2010, the average power from solar was just 5.0 GWh/d1 and the Energy Information Administration (EIA) expects this to be 90 GWh/d in 2016.2 Because space is limited for many photovoltaic (PV) applications, the power conversion efficiency (PCE) of the solar cell is important as cells with higher PCEs generate more power in a smaller area. Currently, the vast majority of commercial PV cells are made with silicon, as these PV cells have proven stability and PCE.3 However, one competing PV technology that has made rapid gains in PCE and received considerable attention over the past few years uses semiconductor perovskites as the light absorbing material.
Perovskites are inorganic complexes that have a crystalline structure similar to that of the mineral perovskite (calcium titanate, CaTiO3). One perovskite complex of particular interest for PV applications is methylammonium triiodoplumbate [(CH3NH3)PbI3, MAIP, (793833)]. Although MAIP was first characterized in 1978,4 it was only recently used in PV applications.5 The optical and electronic properties of MAIP and derivatives containing various combinations of ammonium salts and halides happen to be excellent for use in PV cells.6 Since 2009, PV cells using perovskites of this type have seen a dramatic rise in PCE to the current record of 20.1%.7,8 Although perovskite PV cells may differ slightly in their architecture, a diagram of a typical solar cell is depicted in Figure 1. The use of perovskites in PV cells has been recently reviewed.9,10,11
Early Perovskite PV Cells Using an Electrolyte Solution
Kojima et al. reported the first use of MAIP in a PV cell in 2009.5 In this PV cell, a photosensitive electrode was crafted by depositing MAIP from a precursor solution onto TiO2 coated fluorine-doped tin oxide (FTO). A Pt-coated FTO served as the counter electrode, and an I⁻/I3⁻ electrolyte solution served as the hole conductor, which is a redox couple commonly used in dye-sensitized solar cells (DSSC). Under standard simulated sunlight (Air Mass 1.5 global, AM1.5G)12 and 100 mW/cm2, PV cells using MAIP gave a PCE of 3.81%. Methylammonium tribromoplumbate [(CH3NH3)PbBr3, MABP] was also studied and PV cells made with this material as the photosensitizer had a slightly lower PCE of 3.13%. However, the cell with MAIB exhibited a higher open circuit voltage (Voc) than the one with MAIP (0.96 V and 0.61 V, respectively). In 2011, Im et al. found that by decreasing the thickness of the TiO2 film to 3.6 μm the PCE of a similarly crafted PV cell increased to as much as 6.54% under AM1.5 G light.13 Unfortunately, these devices exhibit very short lifetimes due to the partial dissolution of the perovskite complex by the electrolyte.
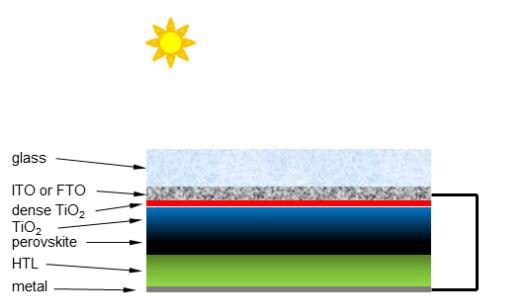
Figure 1: Diagram showing the side view of a typical perovskite based PV cell.
PV cells with spiro-MeOTAD
In 2012, Kim et al. reported that the stability issue of perovskite complexes in electrolyte solutions can be resolved by using a solid state hole transport layer (HTL) such as N2,N2,N2′,N2′,N7,N7,N7′,N7′-octakis(4- methoxyphenyl)-9,9′-spirobi[9H-fluorene]-2,2′,7,7′-tetramine (spiro-MeOTAD, 792071).14 PV cells in this study showed a Voc that is dependent on the TiO2 film thickness (0.6–1.4 μm) and with the thinnest TiO2 film, 0.6 μm, a PCE of 9.7% was obtained. Surprisingly, an added HTL is not necessary for constructing a working perovskite PV cell; Etgar et al. showed that hole conductor-free perovskite PV cells made with a gold counter electrode deposited directly onto MAIP gave a PCE of 5.5% under AM1.5G light at 1000 W/m2 and 7.28% at 100 W/m2.15 Further progress was made in 2012 when Lee et al. reported an all solid state PV cell constructed with a perovskite formed from a precursor solution of PbCl2 and methylammonium iodide (MAI) in N,N-dimethylformamide (DMF) and an HTL of spiro-MeOTAD. This PV cell had a measured PCE close to 8%.16 This study also revealed that the TiO2 n-type semiconductor could be replaced by the electrically-insulating Al2O3, which gave an increase in PCE to 10.9%. Although the active perovskite in this study was referred to as (CH3NH3)PbI2Cl, it was later discovered that the active perovskite in this case was likely chloride-doped methylammonium triiodoplumbate [(CH3NH3)Pb(I3-xClx), CMAIP].17 Although spiro-MeOTAD was an early HTL material used in all solid state perovskite PV cells, other HTL materials have been used as well.18,19,20,21,22
Perovskite deposition techniques
Literature reports suggest that the performance of perovskite-based PV cells depend on the deposition technique used to form the perovskite layer. For example, a PCE of 12.6%–15.0% was accomplished by Burschka et al. by forming the active perovskite layer stepwise instead of from a precursor solution containing both MAI and an inorganic lead source.23 In this study, PbI2 was first deposited onto a 350 nm thin film of TiO2 from a 1 M solution in DMF, and the subsequent reaction with MAI as a solution in 2-propanol formed the active perovskite material. Liu et al. has reported that active perovskite layers can be crafted by simultaneous vapor deposition of PbCl2 and MAI at 10−5 mbar.24 It was found that the cell with the perovskite layer fabricated using vapor deposition yielded a PCE of 15.4% whereas the one prepared using solution processing of PbCl2 and MAI in DMF showed a PCE of 8.4%.25 Vapor deposition of just the ammonium salt MAI onto solution deposited PbI2 has also been reported.26 It was later shown by Jeon et al. that bromide-doped methylammonium triiodoplumbate [(CH3NH3)Pb(I3-xBrx) x = 0.1-0.15, BMAIP]27 deposited as a solution in γ-butyrolactone (γ-BL) containing added dimethyl sulfoxide (DMSO) could give PV cells with a PCE of 16.2%. In this case the formation of a uniform and high density perovskite layer through an intermediate DMSO complex was reported to be important in the high measured PCE.28 These studies have shown that the active perovskite layer can be formed using a variety of techniques and choice of deposition method plays an important role in the performance of the PV devices.
New materials for higher PCE
Various combinations of the component materials used in PV cells have been tried in recent years with the aim of increasing the PCE. In 2014, Zhou et al. report that by using yttrium-doped TiO2 (YTO) as the electron transport layer (ETL), polyethyleneimine 80% ethoxylated (PEIE) modified indium tin oxide (ITO) as the anode, and carefully controlling the humidity during solution deposition of CMAIP yields a device with a maximum PCE of 19.3%.29 However, the average device in their study had a PCE of 16.6%, and it should be noted that the high PCE was measured using a reverse bias scan, which over estimate PCE because of a hysteresis in the current-voltage (J-V) characteristics.30 Excluding the overestimations from only using the reverse bias scan, the best PCE under standard AM1.5G is 17.9% as reported by Jeon et al..8 In this report they used poly(triaryl amine) (PTAA) as the HTL instead of spiro-MeOTAD and the mixed perovskite MABP and formamidinium triiodoplumbate (FAIP) in a molar ratio of FAIP/MABP 17:3 as the photoactive material. In just under 6 years from the discovery of perovskites as viable light absorbing materials for use in PV devices, PCEs of these devices have nearly reached that of low cost silicon PV cells.
REFERENCES
Para continuar lendo, faça login ou crie uma conta.
Ainda não tem uma conta?